Project Summary
Over half of pediatric stroke survivors develop cognitive impairment, limiting their educational attainment and imposing significant financial and emotional burdens on survivors and their families. However, children’s chronic cognitive symptoms are poorly explained by stroke size or location. Instead, clinical neuroimaging studies suggest that stroke in the developing brain may cause long-term changes in uninjured, but functionally or anatomically connected regions that constitute the “stroke connectome.” These changes can even affect white matter integrity in the contralesional hemisphere, and widespread disruption of normal white matter development could contribute to children’s cognitive symptoms. However, the underlying mechanisms altering white matter development outside the infarct are unknown. This knowledge gap prevents the development of targeted therapies to mitigate or prevent the disabling cognitive symptoms experienced by pediatric stroke survivors and their families.
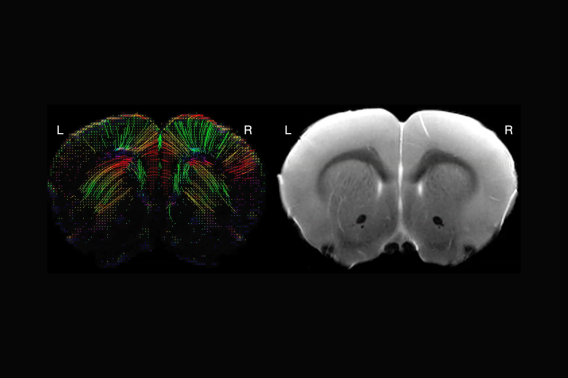
To address this gap, I developed the first model of infarct-induced delayed cognitive decline in “pediatric” mice. My overarching hypothesis is that stroke induces secondary neuroinflammation in uninjured parts of the stroke connectome, and that this chronic inflammation impairs remyelination and developmental myelination, ultimately disrupting white matter in regions outside the acutely infarcted tissue. I have strong preliminary data that juvenile stroke causes chronic neuroinflammation in the striatum, corpus callosum, and corticospinal tract (which are uninjured in this model), and that juvenile stroke mice have significantly less myelin in the corpus callosum and striatum at 8 weeks after infarct. Moreover, this myelin loss is substantially greater in the juvenile brain than the adult brain, implicating a developmentally specific mechanism of white matter injury.
My working hypothesis is that juvenile stroke causes chronic loss or disruption of myelin development in the corpus callosum and corticospinal tract, two regions that are uninjured in this stroke model. Based on my preliminary immunostaining data, I further hypothesize that this white matter loss is greater after juvenile stroke than after adult stroke. I will quantify structural white matter changes in the corpus callosum and internal capsule in juvenile and adult sham and stroke mice at 1 and 7 weeks after surgery using diffusion tensor imaging (DTI).
Project Details
Award Year:
2024
Lead Researcher(s):