By Grace Huckins
Part of our Next-Generation Neuroscience series
Through an unexpected collaboration, Wu Tsai Neuro interdisciplinary postdoc Renzhi Yang discovered that the brain circuits controlling mouse sexual behavior are far more dynamic than researchers had realized.
There are few things simpler than male mating behavior—for mice, that is. Even a male mouse raised in complete isolation will figure out how to mount a female in a matter of minutes. According to conventional scientific wisdom, mouse sex is completely innate: The necessary brain circuits are wired up early in development, and they stay that way for the remainder of the animal’s life.
But Renzhi Yang, a Wu Tsai Neurosciences Institute interdisciplinary postdoctoral scholar, wasn’t so convinced. He had studied neuroplasticity—how neural circuits change over time—as a PhD student at Stanford, and he began his postdoc with psychiatry professor Nirao Shah itching to discover a role for plasticity in innate behavior. He wasn’t just shooting blind: He’d previously found that, while novice males can mate successfully, they don’t do nearly as well as their more experienced counterparts. Plasticity, he thought, could explain that difference.
To pin down a role for plasticity in mouse sexual behavior, however, Yang first had to know where to look. So he teamed up with two of his labmates, postdoc Daniel Bayless and graduate student Chung-ha Davis, to piece together the neural circuit that allows male mice to mate. Bayless and David had discovered that two brain regions, the bed nucleus of the stria terminalis (BNST) and preoptic hypothalamus (POA), both played key roles, but they couldn’t figure out how those regions communicated with each other. Yang gamely took on the task, and that’s when he found something utterly unexpected: Instead of directly activating the POA, BNST instead makes POA neurons more sensitive to activation through a neuroplastic process called long-term potentiation. This genetically determined, supposedly fossilized brain circuit depends on neuroplasticity to function.
The trio’s study was published this August in the journal Cell. Yang spoke with us about his passion for plasticity, his fortuitous discovery, and the priceless power of scientific collaboration.
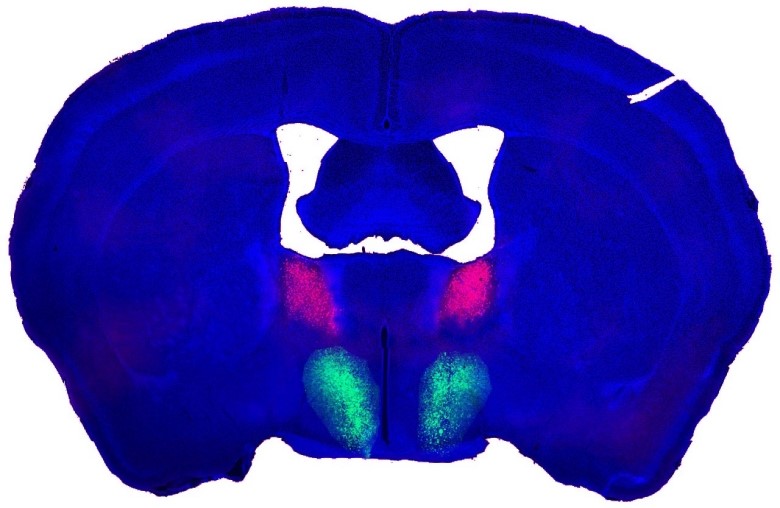
A composite Image of a male mouse’s brain showing the bed nucleus of the stria terminalis (BNST, in pink) and the preoptic hypothalamus (POA, in green). The information is transmitted from pink to green in the neural circuit and drives male mating behaviors.
How did you first get interested in studying mating behavior?
I was actually a PhD student here at Stanford, but I was working in another neuroscience lab—Jun Ding’s lab. During my PhD, I developed a very strong interest in how neuroplasticity mediates behavior changes. We had a collaboration with Nirao Shah [the senior author of the Cell study], and that’s when I learned about innate behaviors. I was working with him on female sexual behaviors, and then I started to think about an interesting question: Are innate behaviors, such as mating and aggression, also regulated by neuroplasticity? That’s what really drove me to join Dr. Shah’s lab for my postdoc.
What do we know about the role of neuroplasticity in innate behavior?
This is a barely touched-on topic in neuroscience. Neuroplasticity is mostly studied in the context of learning and memory, addiction, injury, recovery, et cetera, because these behaviors can obviously be changed by previous experience. By contrast, reproductive behavior does not require any learning—most naive adult male [mice] can perform mating during their first time meeting a female. However, this behavior is actually also highly dynamic and can be modified by experience.
Your recent study is incredibly collaborative—you are one of three co-first authors. How did that collaboration come about?
I wanted to study neuroplasticity in male and female mating behavior, but I was not sure which circuit to work on. Two of my lab-mates, Daniel and Chung-Ha, were working on different brain regions, and then they found out that they are both related to male mating. Daniel found that a specific population in the BNST is essential for sex recognition—it will respond way more actively when you introduce a female mouse into the male mouse cage. And then Chung-Ha, he found a population in the POA, which is downstream of those BNST neurons and can directly drive immediate mating behavior—no matter if the other mouse is female, male, or a toy mouse.
So what was my part? What’s missing here is how the information is transmitted between these two important neural populations. I noticed that the time delay of 10 to 15 minutes from sex recognition to mating initiation seemed to fit the time course of long-term potentiation [LTP, a form of neuroplasticity]. And I found that the synaptic strength of the POA neurons is indeed increased in vivo after the male encounters the female mouse. Substance P [a neuropeptide released by BNST neurons] can increase LTP in the POA neurons, and that’s the mechanism that can lead to activation of this neural population.
Were you surprised to discover such a central role for neuroplasticity in male mating behavior?
I was originally interested in studying neuroplasticity on a long-term timescale. But now it turns out that there is actually relatively short-term neuroplasticity happening right in this circuit. And it’s a really good paradigm to study neuroplasticity. What’s really nice about innate behavior is that it’s so robust. With learning and memory, you need so many animals to see a significant result, you need to train them extensively, and the correlation between neuroplasticity and behavior is usually not that obvious. But in this case, if you block LTP by blocking substance P, there’s no mating. I feel very lucky to have gotten involved in this study.
Could this short-term neuroplasticity play a more general role in the brain, beyond just mating behavior?
[This research] provides a really novel mechanism for neuronal activation in the timeframe of tens of minutes. This mechanism can potentially function as a molecular clock that can delay a behavior or response. So in all these situations where animals don’t want to make immediate decisions or immediate movements, but spend some more time assessing the environment or integrating other information, in those situations, this kind of neuropeptide-mediated LTP mechanism may [be at work]. So in the future, when I see similar behavior, whether it’s innate behavior or other behaviors, I would check if this is one of the mechanisms that’s involved.
Did the success of this project reinforce for you the importance of scientific collaboration?
Definitely. I wish scientists across different labs could talk with each other more. Can you imagine if Daniel and Chung-ha and I were in different labs? This project will not have come into existence at all, and I would never get the idea to study LTP in the signal transmission between BNST and POA. So it really inspired me to try to communicate more with other scientists.
