Unraveling how seizures change brain insulation

Magnetic resonance imaging, or MRI, is a powerful imaging technology that’s widely used in the clinic to take detailed, three-dimensional images of soft tissues.
When you sprain an ankle, a doctor might order an MRI to visualize the inflammation in your tendons, ligaments, and muscles. MRIs are also ideal for studying the detailed structure of the brain, because they don’t emit harmful X-ray radiation and are able to capture multiple aspects of brain anatomy with exquisite contrast. Researchers are continually expanding the limits of this powerful technology and broadening its applications.
Gustavo Chau Loo Kung, a Stanford Interdisciplinary Graduate Fellow and member of the Center for Mind, Brain Computation, and Technology (MBCT) at the Wu Tsai Neurosciences Institute, is using experimental forms of MRI to investigate how myelin, the protective layer that covers every nerve cell in the brain, changes with repeated seizures.
Akin to the insulation that covers electrical wires, myelin is the body’s way of sheathing nerve cells to protect and accelerate the rate of electrical firing. Unlike electrical insulation, however, we now know that the myelin sheath is not static. Rather, the myelin layer is regulated by the same electrical activity it facilitates. This means that nerves that experience more electrical firing—for instance, the nerves that are activated during a seizure event—become more heavily myelinated.
This concept, called adaptive myelination, is a focus of Chau’s research in the laboratory of Jennifer McNab in the Stanford Medicine Department of Radiology. Chau is also collaborating closely with Juliet Knowles’ newly established lab in the Department of Neurology. Much of his research relies on the Neuroscience Preclinical Imaging Laboratory, Wu Tsai Neuro’s cutting edge community laboratory for experimental MRI research.

Chau running an MRI experiment at the Neurosciences Preclinical Imaging Laboratory at the Wu Tsai Neurosciences Institute. Image courtesy Gustavo Chau.
We spoke with Chau about his research on how epilepsy changes the myelination state of the brain and the importance of interdisciplinary research. (Learn more about his SIGF project.)
Can you tell us a little about your path to Stanford and your current work?
I’m originally from Peru, and I completed both my bachelor’s and my master’s degrees in Electrical Engineering there. I’ve always been interested in applying electrical engineering techniques to medicine and other clinical uses, and during my master's I started to explore the field of neuroscience. That's what brought me to Stanford—I knew this was a major collaborative center for neuroscience research, and I wanted to use my technical background in brain-related work.
I’ve been at Stanford since 2018, working on my PhD in Bioengineering. My research focuses on using quantitative magnetic resonance imaging, or MRI, to probe different structures in the white matter of the brain. Specifically, I use MRI to image the entire brain and study how myelination changes during the development of some types of generalized epilepsy.
Can you tell us more about the goals of your work, and the tools you use?
The main goal is to try to understand the effects of a newly discovered mechanism called activity-dependent myelination in conditions like epilepsy. We hope that by understanding how these myelination changes happen and what role they play in seizures in animal models, we will be able to find better targets to treat seizure-related disorders. In addition, because the tools we’re using are non-invasive, the hope is that we can translate our research techniques to human studies and perform some of this work in patient cohorts. This will allow us to better probe how different treatments are affecting the structure of myelination, not only in epilepsy but maybe in other diseases as well.
You’re using new forms of MRI imaging in your work—can you tell us more about how these technologies are different than what is currently used in the clinic?
The technological building blocks used in MRI have existed for quite a bit of time already. What we are doing in the lab is looking in detail at the brain’s white matter using a combination of MRI techniques and analyses that are not in clinical use. Basically, from our MRI images, we estimate the g-ratio, which is the ratio of the inner axon diameter (without its myelin sheath) divided by its entire diameter (including myelin). When we combine the MRI measurements with biophysical models, we can get some ideas of how myelination is changing over the course of the development of generalized seizures.
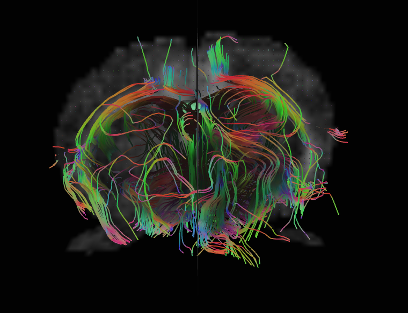
Diffusion MRI data can reveal likely pathways of nerve bundles traveling through the brain. Advanced techniques can now measures how well insulated different nerve bundles are, which affects the speed and strength of connections between brain regions. Image courtesy Jieun Kim, Neuroscience Preclinical Imaging Laboratory.
How do you see your work advancing epilepsy care?
That's an interesting question! One way in which I see this research contributing to clinical care is in furthering our understanding of how the aberrant electrical activity of repeated seizures affects the brain long-term, and what that might mean for epilepsy patients. Secondly, at the conclusion of this project we’d hopefully have a more quantitative measure of how the brain changes in the context of epilepsy, which could give clinicians a better idea of how treatments are working and how seizures are evolving or affecting the brain.
Your background includes a fair amount of data science and computational analysis. How do those skills get incorporated into your current work?
The biggest concepts I use from my electrical engineering background are signal processing and image processing, which are super useful for MRI, along with Fourier theory. Also, the ability to curate data, design experiments, and think about methodology has also carried over into my neuroscience work. Recently, I've been working on new ways to obtain better measurements of axon diameters from MRI images, using machine learning. My data analysis background has really been helping me there. Additionally, I already had experience with the reality of research—how to be resilient when experiments fail, and how to keep coming up with new ideas and continue doing the work.
What makes the Wu Tsai Neurosciences Institute community special?
The interdisciplinary nature of the Wu Tsai Neuro community has really impacted my work. For example, the project I’ve been describing here was conceived during an institute retreat, where my PI [Jennifer McNab] presented a talk on how our lab uses MRI to map axon diameters. Afterwards, a few other professors—Michelle Monje, John Huguenard and Juliet Knowles—approached her to chat, because it turned out they’d been looking for some kind of non-invasive measurement of axon diameter for their work in a mouse seizure model. Normally, to measure myelination in mice you’d have to sacrifice the mouse, section the brain, and perform electron microscopy images over tiny pieces of it in order to get a usable image. With MRI, the mouse stays alive and can be re-measured, and you can also get whole-brain imaging coverage, which is a big benefit.
In addition, I learn a lot in the seminars and the symposiums that are organized by Wu Tsai Neuro and the Center for Mind, Brain, Computation, and Technology (MBCT), and the funding of the MBCT program has allowed me to travel to conferences, share my work, and get to know other people in the field. And of course, I’m so thankful for the Stanford Interdisciplinary Graduate fellowship I received–it’s given me a lot of freedom in how I approach my research.