Brain imaging and stimulation technologies receive 2025 Neuroscience:Translate awards

Three teams developing promising neurotechnologies with the potential for tremendous impact on human wellbeing have been named recipients of the 2025 Neuroscience:Translate awards from the Wu Tsai Neurosciences Institute at Stanford.
The awards program supports cross-disciplinary Stanford research teams who are developing new devices, diagnostic procedures, software, pharmaceutical therapies and other products that can be brought rapidly to market through new startup companies or partnerships.
Wu Tsai Neuro has awarded Neuroscience:Translate grants annually since 2019, in partnership with the Stanford Musallem Center for Biodesign. In addition to funding translational research and development work, the program connects teams with Stanford Biodesign's network of industry mentors—some of whom serve directly on the program's oversight committee—who share strategic advice and connections to help advance winning teams’ discoveries from the lab to the clinic.
"The Neuroscience:Translate program is intended to support and mentor high-potential projects coming out of Stanford research labs and accelerate their path to practical applications for patients," said Gordon Saul, the executive director of Stanford Biodesign. “Since starting the program in 2019 we have seen strong growth in both the number of high-quality projects applying as well as in funded projects making significant progress into clinical trials to advance patient care.”
"These grants facilitate the challenging transition from basic research to application, by providing both funding and mentorship. Our goal is to see Stanford discoveries move into practice through licensing, spinoff companies, and clinical trials,” said Allison Okamura, a Wu Tsai Neuro deputy director and Neuroscience:Translate grant committee member who is the Richard W. Weiland Professor in the School of Engineering.
This year’s awardees reflect tremendous progress that neuroscientists have made in recent years in two long-standing goals of the field: the ability to sensitively image the sources of brain disorders in patients with neurological and psychiatric disease, and the ability to noninvasively nudge dysfunctional brain circuits back toward healthy function.
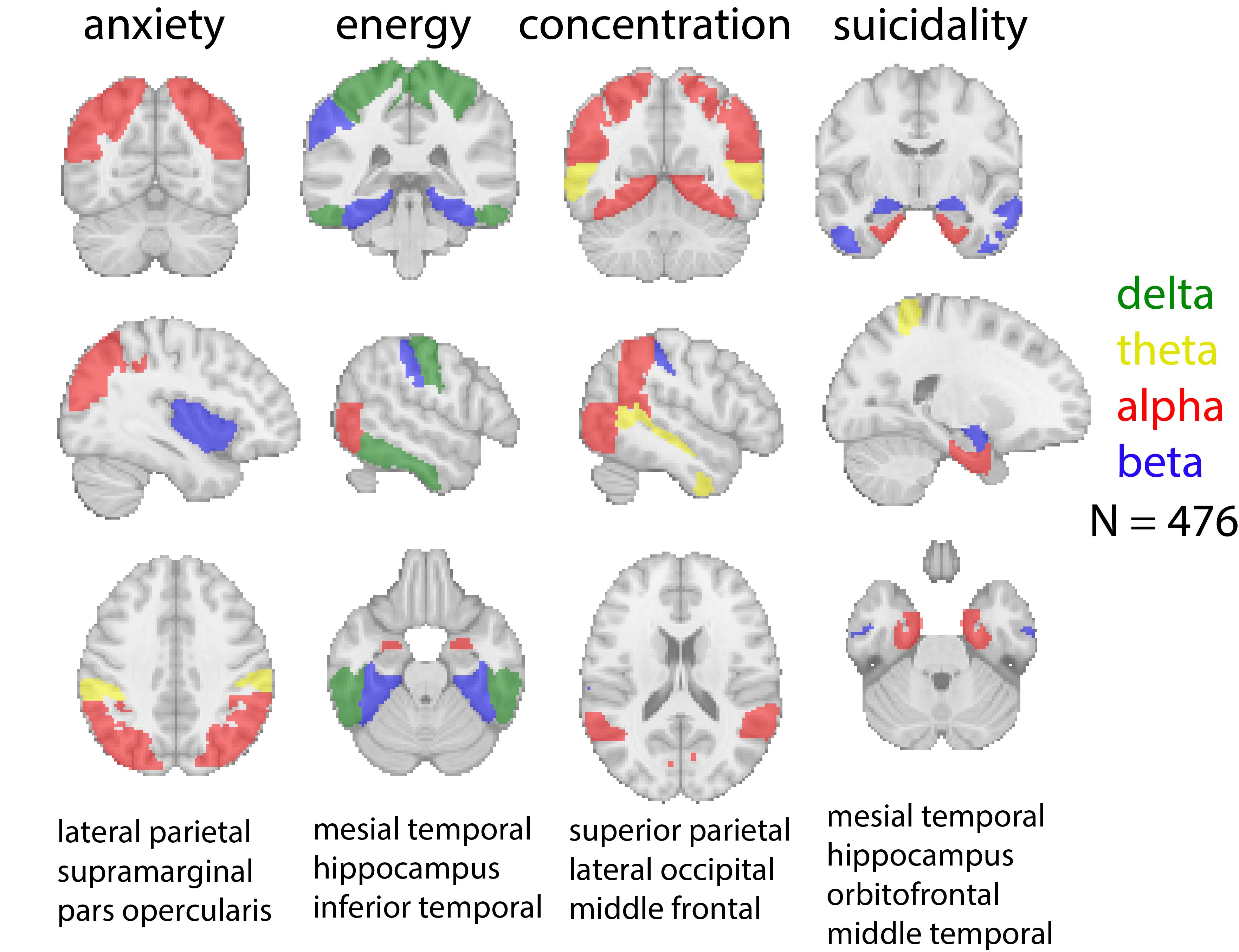
One awarded project has developed a powerful software package that will enable clinicians to better discern patterns of activity deep in the brain associated with clinical depression. Electroencephalography (EEG) is a widespread technology for monitoring brain activity, but has historically been limited in how deep it could peer into the brain—limiting its ability to detect problems lurking below the surface.
But new software developed by the Stanford research team gives existing clinical EEG systems new superpowers—potentially allowing clinicians to develop individually targeted treatment plans for patients based on brain-wide patterns of activity associated with their symptoms. The team aims to use their Neuroscience:Translate award to accelerate the development of their software—called EEG-IntraMap—into a widely accessible, clinically-validated platform for psychiatry.
“EEG-IntraMap has the potential to fundamentally change how we treat depression,” said Corey Keller, an assistant professor of psychiatry and behavioral sciences at Stanford and the Veterans Affairs Palo Alto Health Care System, who oversees the project with Scott Linderman, a Wu Tsai Neurosciences Institute faculty scholar in the Department of Statistics. “By giving clinicians a window into deep brain activity using standard EEG equipment, we could move away from trial-and-error approaches and toward precision psychiatry that gets patients the right treatment faster. ”
“While neurosurgical conditions benefit from tools like deep-brain stimulation or brain-computer interfaces, mental health conditions lack noninvasive brain measurement tools that can reliably guide clinical decisions,” added Ajay Subramanian, a postdoctoral scholar in Keller’s Precision Neurotherapeutics Lab who spearheaded the development of EEG-IntraMap during his PhD work in the lab. “My biggest hope is that this work leads to an accessible brain scan that helps clinicians develop better warranted and even personalized mental health treatment plans for their patients.”
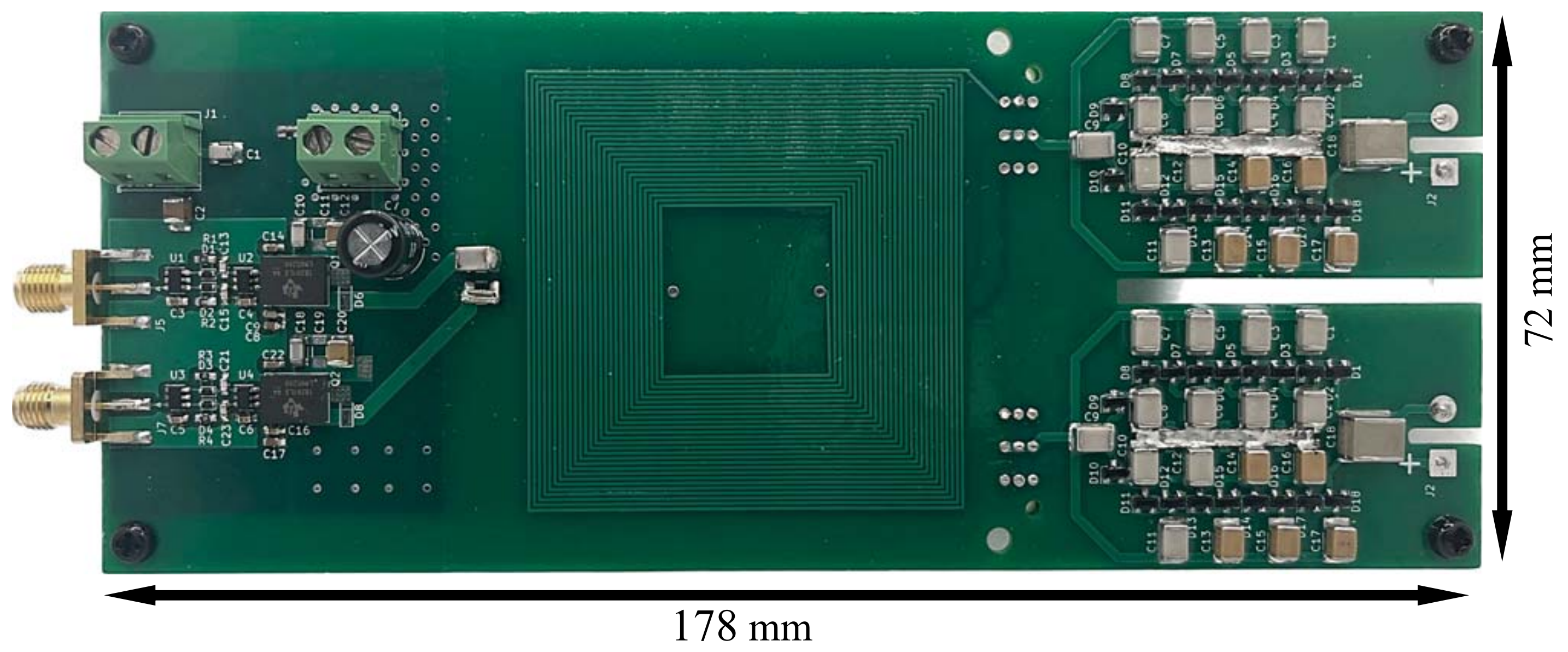
Another newly funded project aims to dramatically increase patient access to non-invasive clinical brain stimulation—by developing a smaller, lighter, and cheaper form of transcranial magnetic stimulation (TMS) technology.
In the hands of project co-lead Nolan Williams, TMS has been effective at reducing symptoms of severe clinical depression by 80%. However, the technology has remained cumbersome, expensive, and restricted to a few clinical centers around the world. Now, in collaboration with the Stanford University Power Electronics Research Lab, led by Juan Rivas-Davila, these world experts in brain stimulation and electronics design aim to bring that transformative treatment to the masses.
“Current TMS devices are difficult for psychiatrists to incorporate into their routine practice,” explains Derrick Buchanan, a postdoctoral research fellow in the Brain Stimulation Lab and member of the project’s neuroscience team. “Our vision sees TMS as a tool that is accessible to any psychiatrist. Our cost-effective, portable device is a turnkey solution for any psychiatrist to begin using TMS in their clinic.”
“I'm excited to work with the Brain Stimulation Lab to create something that combines our areas of expertise and improves access to TMS depression treatment,” added Malachi Hornbuckle, a PhD candidate in the SUPER Lab and member of the project’s engineering team.
A third project—focused on imaging inflammation in the brain—won an additional year of funding to extend the impressive progress made with their 2024 Neuroscience:Translate award.
The research team—led by Michelle James, an assistant professor of radiology and of neurology and neurological sciences, and Hannes Vogel, professor of pathology and of pediatrics—has been developing and testing a sensitive PET radiotracer test to distinguish between harmful (pro-inflammatory) and helpful (anti-inflammatory) immune cells in patients with multiple sclerosis (MS), a critical unmet need that could help speed the selection of the right therapy for a given individual. With an additional year of funding, they will deepen and extend the applications of this work to Alzheimer’s disease and other disorders linked to nervous system inflammation, and advance the translation of this tool towards clinical application.
2025 Neuroscience:Translate Projects:
Development of a novel compact, portable, ruggedized transcranial magnetic stimulation device
- Nolan Williams (Psychiatry and Behavioral Sciences)
- Juan Rivas-Davila (Electrical Engineering)
EEG-IntraMap: Accessible deep brain insight for precision depression care
- Corey Keller, (Psychiatry and Behavioral Sciences, Veterans Affairs Palo Alto Health Care System)
- Scott Linderman (Wu Tsai Neurosciences Institute, Statistics)
Clinical translation of novel PET radiotracers for mapping innate immune activation in CNS diseases (Renewal)
- Michelle James (Radiology, Neurology and Neurological Sciences)
- Hannes Vogel (Pathology, Pediatrics)