Neuronal and synaptic genes expanded in size and diversity during evolution
How did nervous systems, with their incredible complexity, evolve across different species?
New research supported by the Wu Tsai Neurosciences Institute’s Interdisciplinary Postdoctoral Scholars program zeroes in on the surprising observation that many genes found in brain cells and synapses — the points of communication between neurons — are among the largest in the animal kingdom.
These giant neuronal genes span hundreds of thousands of base pairs of DNA, and are often counted among the genes implicated in neurological disorders when they become mutated or misregulated.
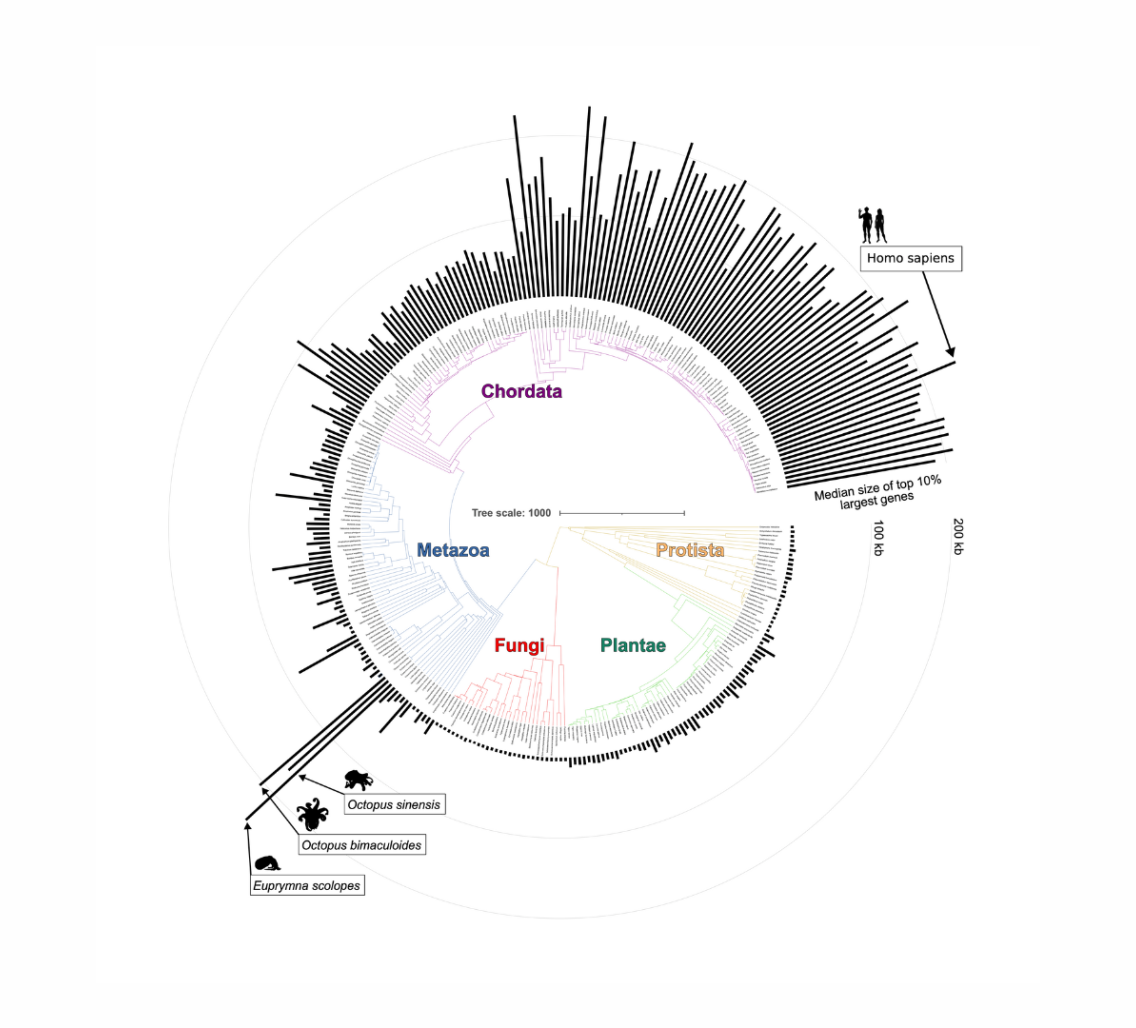
To uncover general principles of how these large genes evolved alongside nervous systems, Wu Tsai Neuro Interdisciplinary Postdoc Matt McCoy compared the sizes of genes in different species that originated from a common ancestor across a wide range of Earth's tree of life. This comparison revealed a distinct class of large genes that existed before the diversification of animals and, in many cases, even before the emergence of neurons as specialized cell types.
The research was published March 8, 2024 in Current Biology.

Wu Tsai Neuro Interdisciplinary Postdoctoral Scholar Matt McCoy. Image credit: Steve Fisch.
Tracing the evolutionary journey of these ancient large genes, McCoy and advisor Andrew Fire, the George D. Smith Professor of Molecular and Genetic Medicine and professor of pathology and of genetics, found comparable giant genes in both humans and in cephalopods such as squids and octopuses.
Cephalopods, which are closely related to snails and slugs, are about as far as you can get from humans in the animal kingdom, but separately evolved their own highly developed nervous system and marvelously complex behavior. The fact that giant genes evolved separately in both of these lineages suggests a surprising general role for gene size in the development of complex nervous systems in multicellular animals.
This is particularly true because these genes have grown larger and developed more variants in animals during evolution despite being under strong so-called “purifying selection”—a process that removes harmful mutations to maintain genetic stability. The authors suggest that genes’ growth and diversification may have provided the molecular flexibility needed for complex nervous systems to evolve.
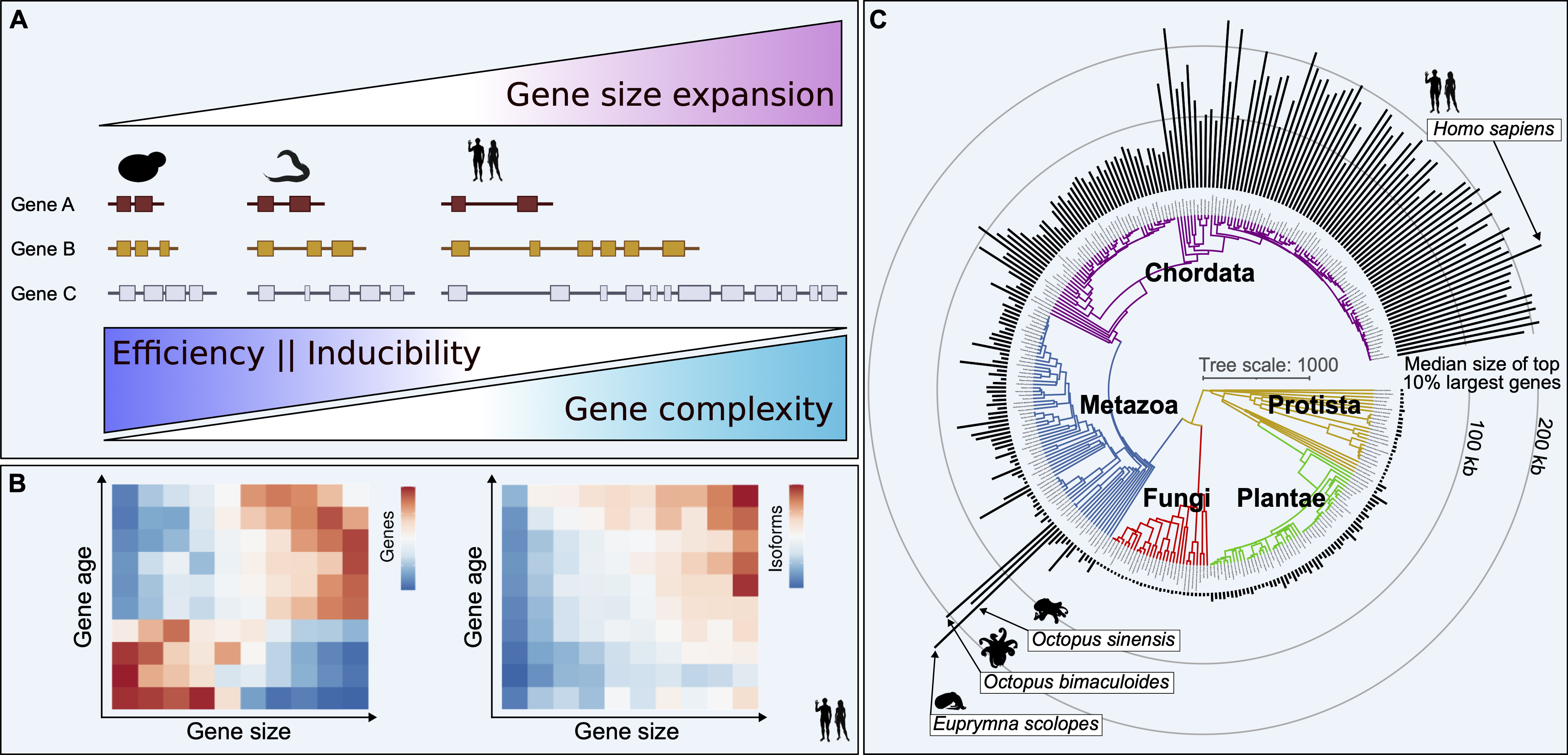
McCoy and Fire propose a new model for how the size of genes changes and what this means for complex organisms: (A) When the total size of an organism's genome increases, the parts of the genes that don't code for proteins (introns) also grow. This affects the overall size of the organism's genes. Bigger genes can do more complex tasks, but this complexity makes it harder and less efficient for the gene to be turned on or off. (B) Genes that have been around for a very long time tend to be larger and, as a result, more complex. (C) The differences in gene size between species can lead to significant variations in how complex their genes and traits (phenotypes) can be. Image credit: Matthew J. McCoy and Andrew Z. Fire.
This research offers a new perspective on how inherent genomic properties like gene length may have contributed to the evolutionary development of complexity in the animal kingdom. By understanding these genomic underpinnings, McCoy and Fire argue, we can gain insights into both the origins of nervous system complexity and the genetic basis of neurological diseases.



