Project Summary
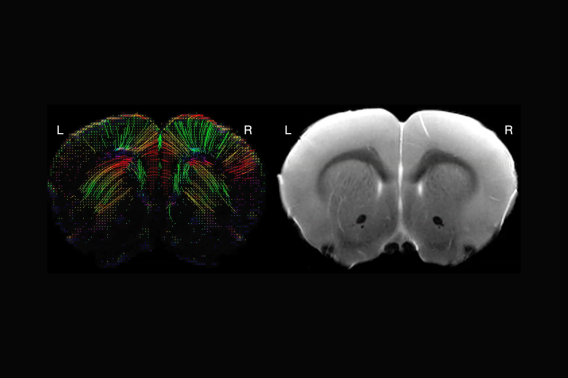
In epilepsy, a disease affecting 1% of all children, brain networks undergo maladaptive change (plasticity) and become predisposed to seizures. In the 30-40% of children with epilepsy who have medication-resistant seizures, the seizures become more frequent and severe over time, with concurrent loss of cognitive ability. A better understanding of the process underlying such epilepsy progression is required to develop improved treatments. A newly discovered form of brain plasticity is activity-regulated myelination, in which neuronal activity drives the formation or adjustment of myelin. Myelin sheaths coat neuronal axons and control the speed and synchrony of communication between neurons. Activity-regulated myelin plasticity is important for multiple forms of learning and cognition. However, my lab demonstrated that generalized seizures (seizures involving both sides of the brain) can induce aberrant myelination of the affected brain network, which in turn worsens seizure severity. We previously found that in mice with seizures, myelination is abnormally increased in large regions of the brain that correspond to the seizure network. We now propose new studies using MRI to map myelination in mice with epilepsy and pharmacological seizure treatment initiated before or after seizures have progressed (Aim 1), or in mice with epilepsy and genetic suppression of myelin plasticity (Aim 2). The proposed experiments will enable us to prove whether seizures cause the observed myelin changes, whether myelin changes are reversible, and the underlying cellular and molecular mechanisms. The information gained from these studies will critically inform efforts to develop treatments that prevent maladaptive myelination and epilepsy progression.
Project Details
Funding Type:
Neuroimaging Pilot Grant
Award Year:
2024
Lead Researcher(s):