Exploring MRI's role in neuroscience research on model organisms
Magnetic Resonance Imaging (MRI) is an essential tool in neuroscience. It allows researchers to delve deep into the brain, analyze neural anatomy, and map complex brain functions with precision. In addition to its more familiar use in human research, MRI is also invaluable for basic and translational research in rodents and other model organisms. Despite the technique’s central importance, its reliance on the principles of electromagnetism and large, expensive equipment can be daunting to those new to the field.
Recognizing the potential for wider application in small-animal neuroscience research, the Neurosciences Preclinical Imaging Lab (NPIL) at the Wu Tsai Neurosciences Institute hosted its third annual symposium. The event showcased the capabilities of MRI in supporting research on model organisms and highlighted the latest advancements and applications in MRI technology.
As one of the seven Neurosciences Community Laboratories within Wu Tsai Neuro, NPIL equips scientists with cutting-edge MRI technology and expertise. Led by Jieun Kim, NPIL director, the lab supports a wide range of preclinical imaging applications in diverse animal studies. Kim encourages neuroscientists of all experience levels to consider incorporating MRI into their research projects to enhance insight and discovery.
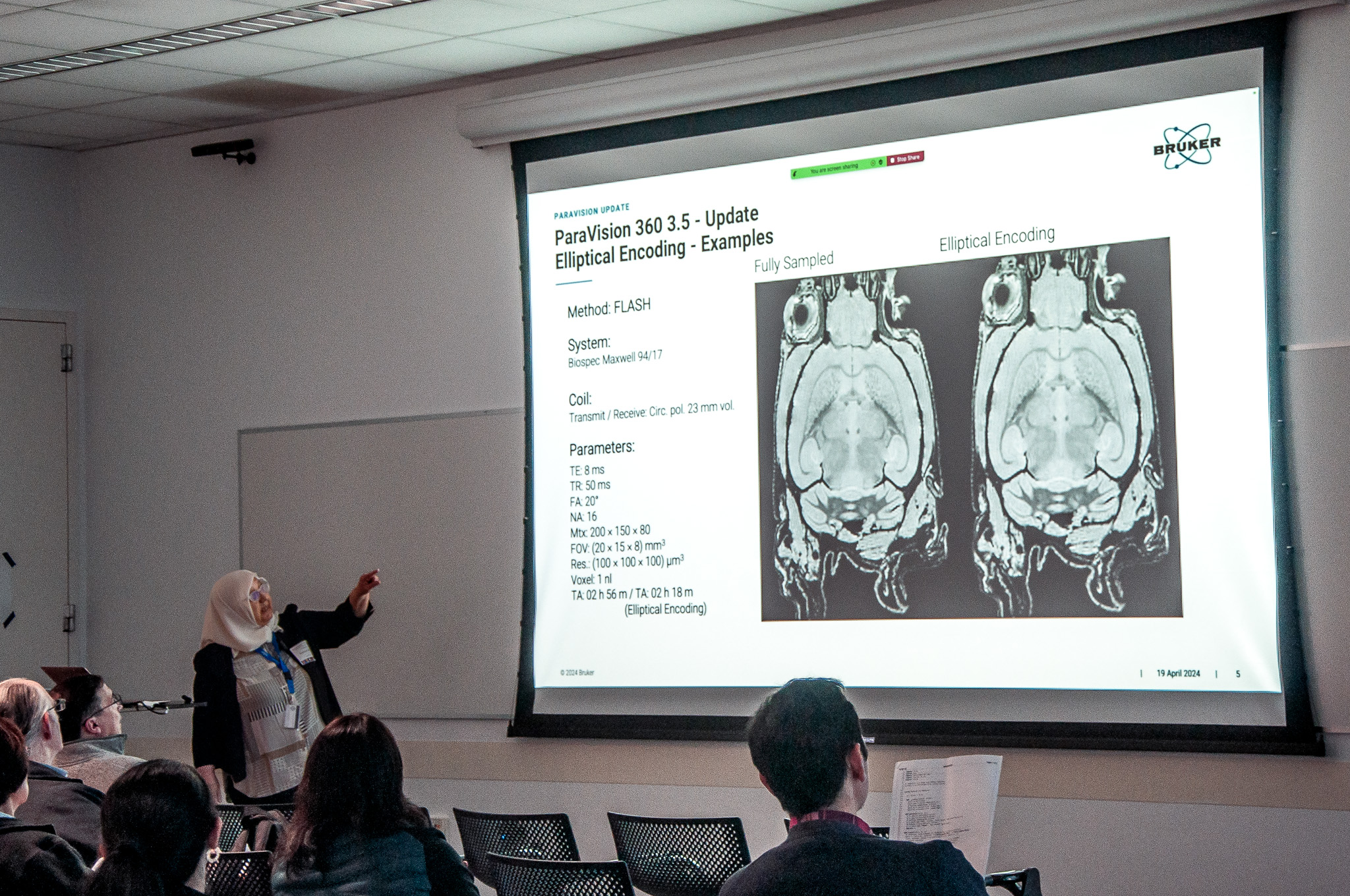
After NPIL Faculty Director Jin Hyung Lee provided a brief overview of the lab’s origins, Saaussan Madi, a senior applications scientist from MRI manufacturer Bruker, a co-sponsor of the event, explained the new features of the NPIL’s unique 7-Tesla large-bore preclinical MRI magnet.
Madi’s presentation outlined recent software enhancements that have significantly improved its capabilities. These updates streamline imaging processes by reducing image distortion and scan times, and introduce new capabilities for detailed neurotransmitter analysis and sophisticated data visualization.
Highlighting the collaborative potential of MRI in small-animal neuroscience research, a panel discussion on "MRI for Basic Neuroscientists" featured leading preclinical imaging scientists from Bruker, neuroimaging experts from Stanford, and the National Institutes of Health.
The panelists discussed the versatile applications of NPIL's advanced MRI technology, the comprehensive services provided by NPIL, and effective data management practices. They also offered insights on how neuroscience researchers at all experience levels, including those new to MRI, can leverage NPIL resources to enhance their studies.
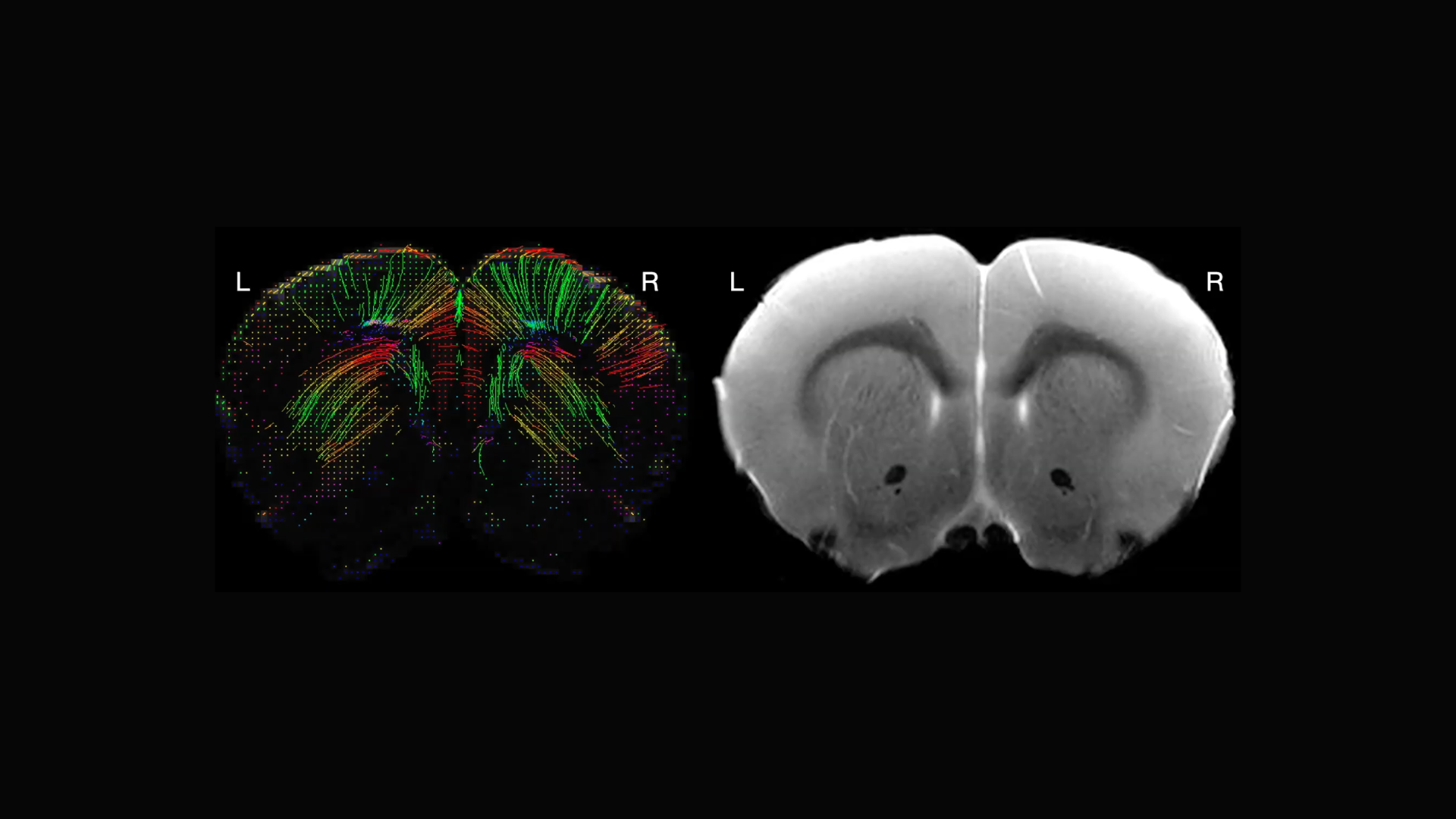
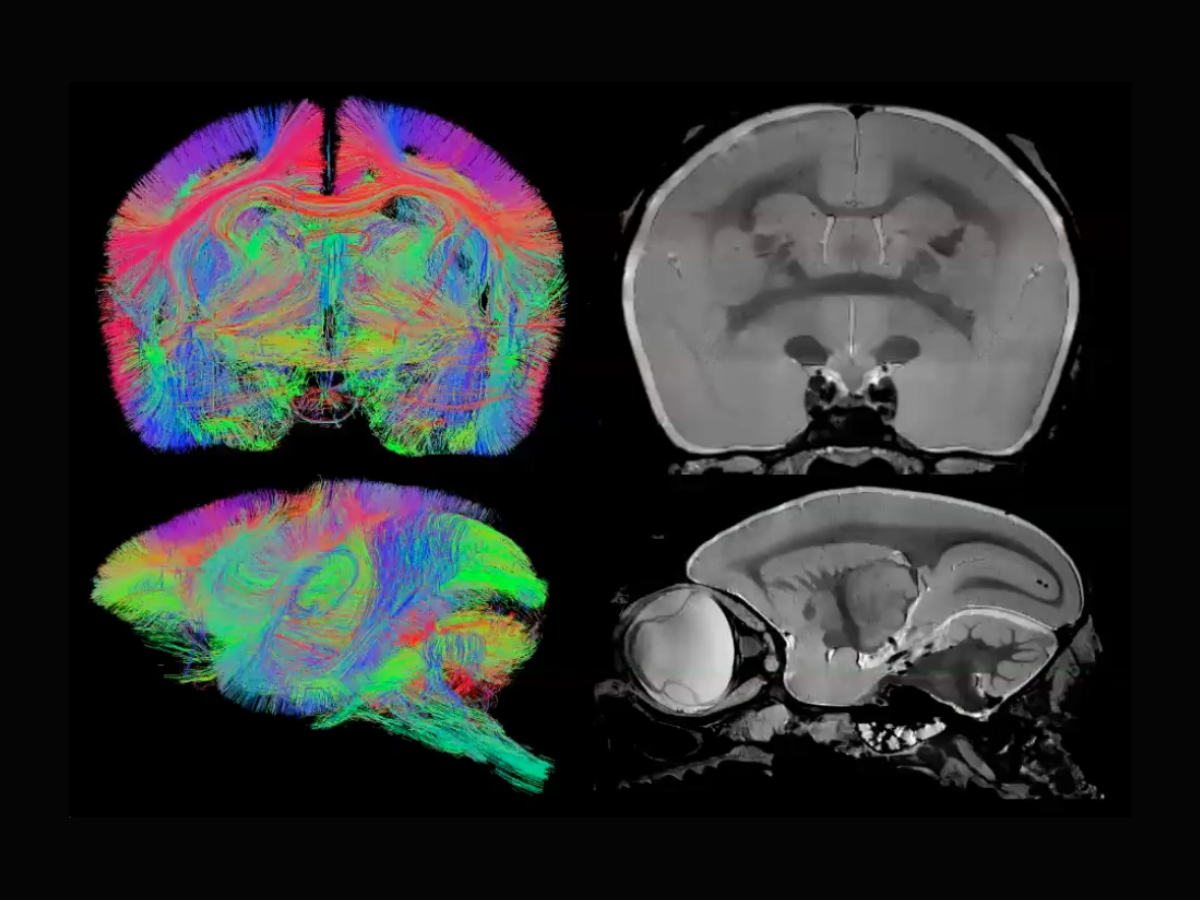
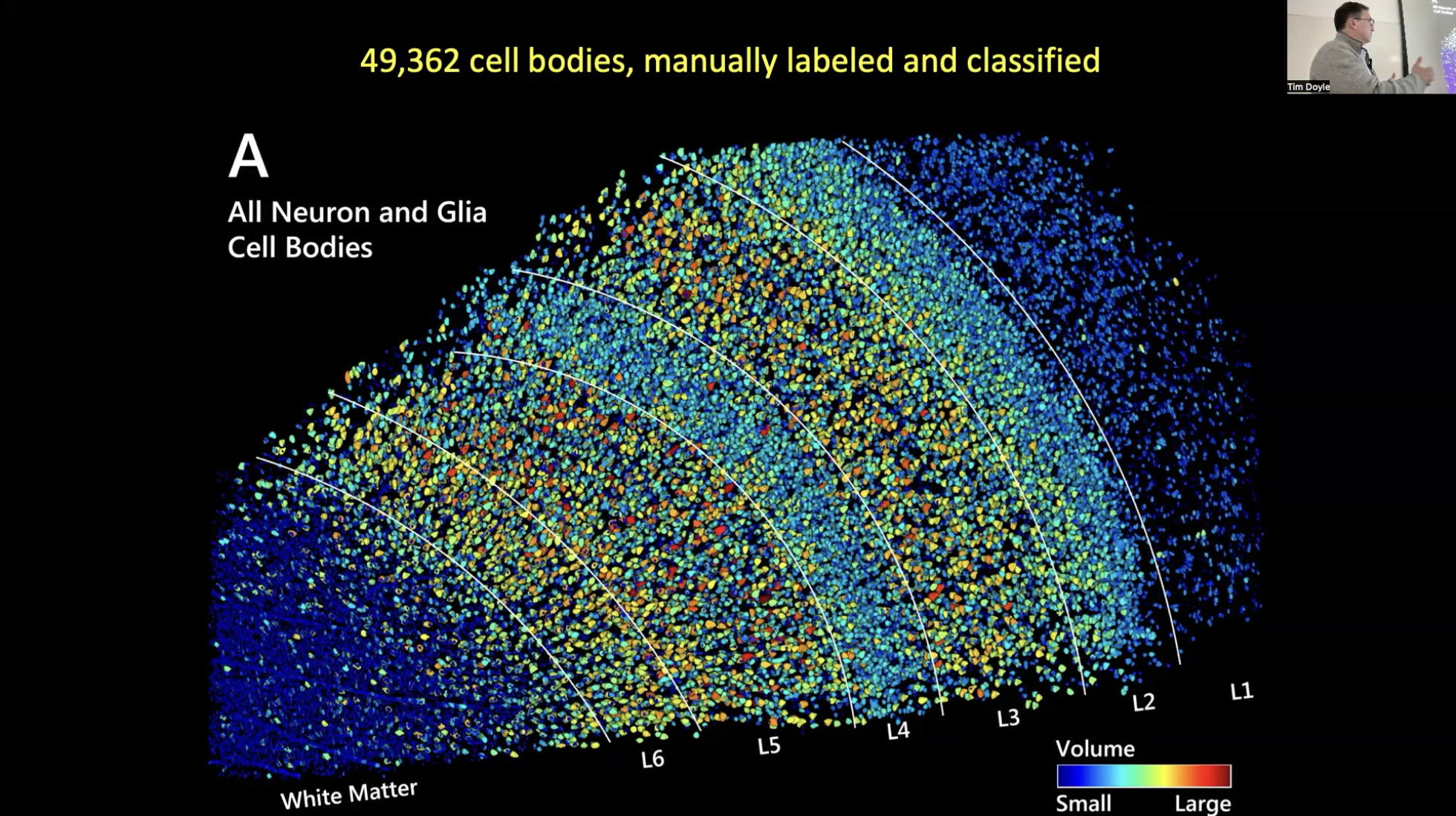
Keynote speaker Peter Basser, Senior Investigator at the National Institute of Child Health and Human Development (NICHD) in the Section on Quantitative Imaging and Tissue Sciences, showcased the capabilities of mesoscale imaging in MRI. This technique reveals intricate brain structures such as cortical layers and nuclei, often invisible in conventional scans.
Basser highlighted its broad applicability, providing examples of how it benefits various neuroscience and clinical disciplines. He discussed the current challenges and potential advancements in mesoscale MRI, including the need for improved voxel (3-D pixel) resolution and more effective experimental designs. Basser noted that innovations in high-field, high-gradient MRI could revolutionize both pre-clinical and clinical applications, bridging significant gaps in neuroscience research.
NPIL awards annual Pilot Grants to support new researchers in the field or enable novel or experimental studies by more experienced researchers. Four of the 2023 NPIL Pilot Grant Awardees presented the progress they had made in their research projects at the 2024 Symposium.
Sedona Ewbank, a neuroscience PhD student and NSF Graduate Research Fellow working in the laboratory of Raag Airan in the Department of Neurosciences, used a technique called multi-shell diffusion-weighted imaging to study structural changes induced by ketamine in the brains of mice. Her research aims to quantify these changes, explore variables that might affect ketamine-induced structural plasticity, and investigate its potential to reverse tissue microstructure deficits in genetic disease models.
Vanessa Doulames, a research scientist in Sarah Heilshorn's Biomaterials Group in the Department of Advanced Materials, presented her research on therapies to repair spinal cord injuries by guiding pluripotent stem cells to regenerate damaged tissue in rat models. Her project aims to refine cell implantation techniques, increase the sample size, and conduct comprehensive analyses of various experimental groups.
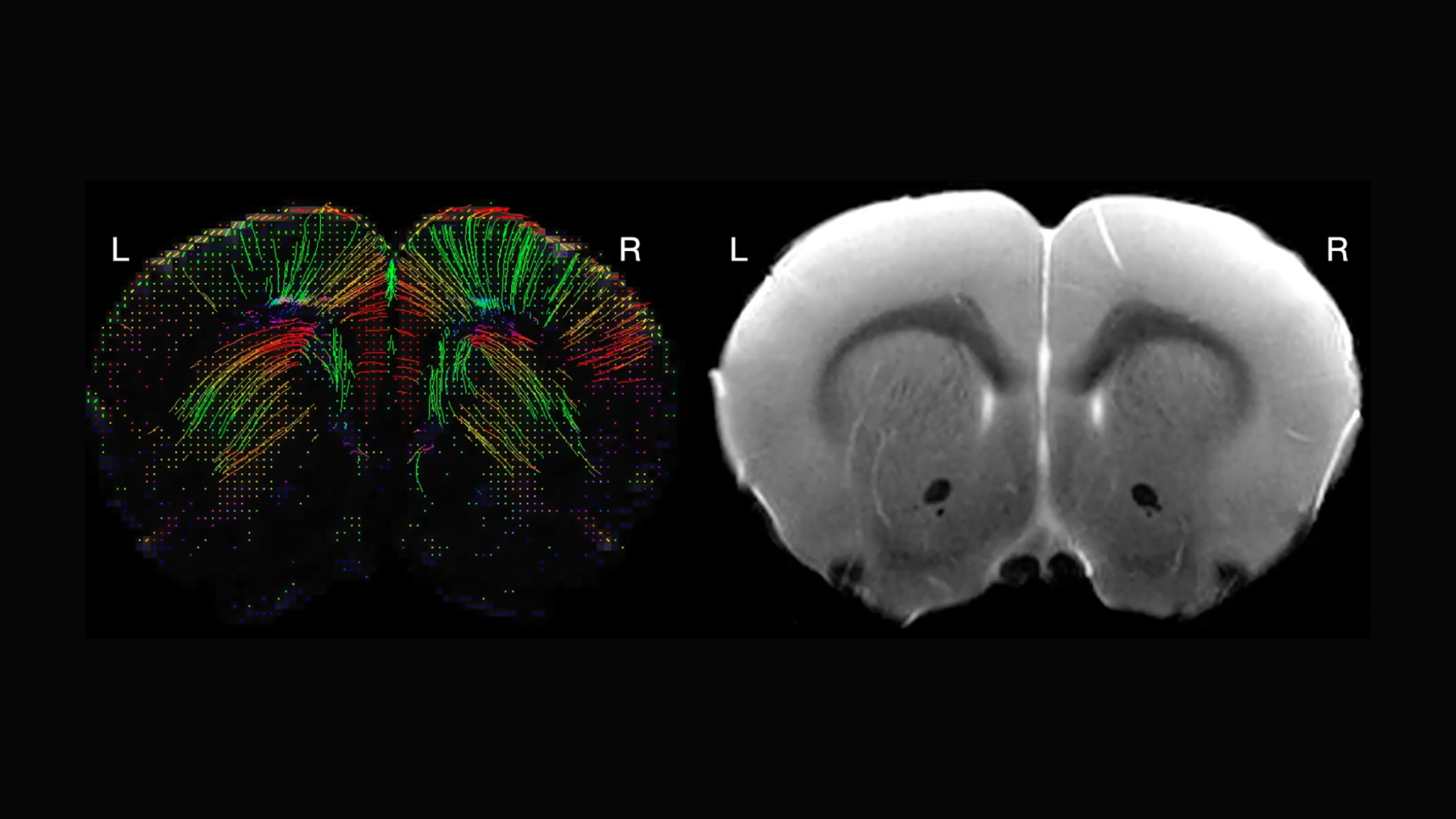
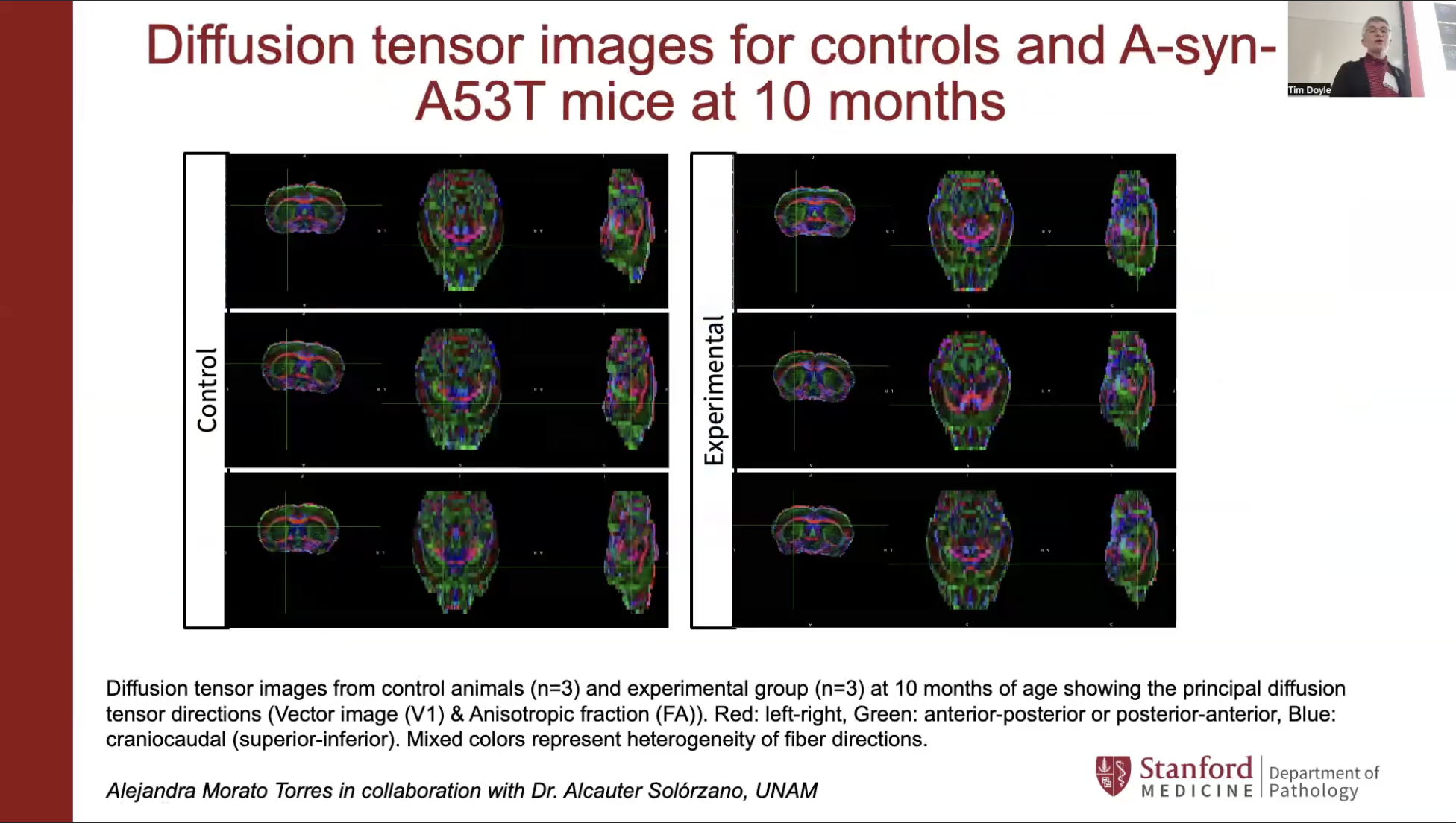
Birgitt Schuele, an associate professor of pathology at Stanford, shared her work on the role of alpha-synuclein — a key protein in Parkinson’s disease — using a rodent model to examine how gene dosage affects behavior, brain activity, structural connectivity, and metabolites. Her NPIL-funded research aims to provide deeper insights into the disease’s mechanisms.
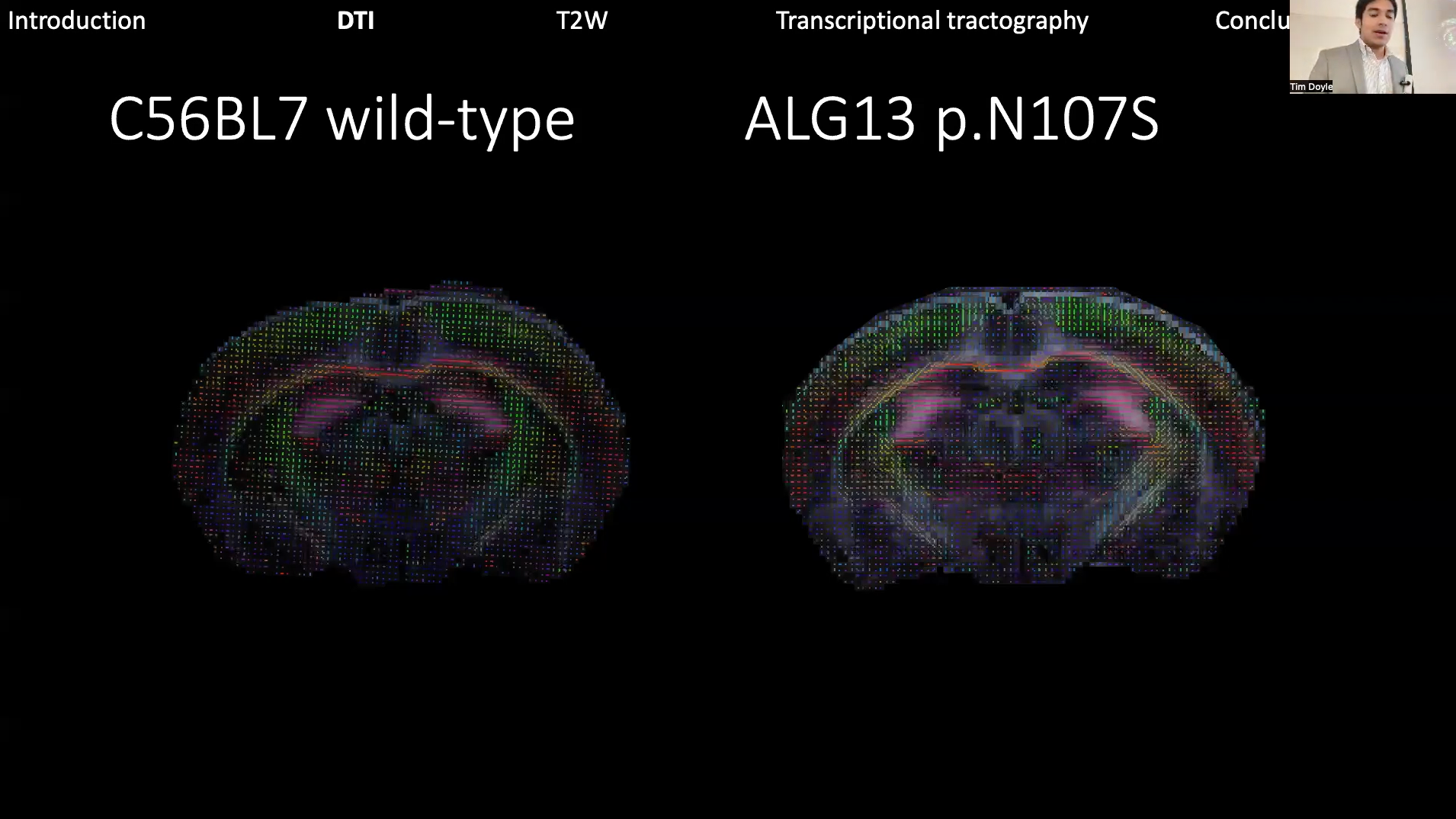
Richard Coca, now a PhD student at Boston University who started his research as a Stanford undergraduate in the Wheeler Lab, described his NPIL research exploring the impacts of the ALG13 gene mutation using a novel mouse model. His project utilized neuroimaging to understand how this mutation contributes to epilepsy and other symptoms, with the long-term goal of translating these findings into clinical practice.
After the 2023 Pilot Grant recipients shared their findings, Tim Doyle, Director of the Neurosciences Community Labs at Wu Tsai Neuro, and Jin Hyung Lee, associate professor of neurology, neurosurgery, and bioengineering and the NPIL’s faculty director, announced the 2024 NPIL Pilot Grant Awardees.
The 2024 NPIL Pilot Grant projects are led by scientists from various departments across the School of Medicine and Stanford Health Care, including microbiology, immunology, neurosurgery, neurology, and pediatrics (see below).
The symposium concluded with a poster session where attendees networked and discussed how to integrate the day’s insights into their research. Refreshments were served as scientists exchanged ideas and planned collaborations. To close, Kim encouraged attendees to visit NPIL for more discussions and a tour of the facilities.
Funded projects
Brain response to influenza virus infection in the lung
- Yueh-hsiu Chien, Microbiology and Immunology, SOM
This project aims to find how the brain regulates the immune system during influenza virus infections, focusing specifically on the role of glucocorticoids (GC), which are essential for managing immune and physiological responses. The study will employ MRI technology to investigate how influenza-induced hypoxia—low oxygen levels—affects the brain in mice. Ultimately, this research seeks to uncover the mechanisms behind glucocorticoid dysregulation during influenza, potentially enhancing our understanding of immune responses in viral infections.
Decoding stem cell-induced recovery in time and space: a longitudinal DTI and MRS study to assess microstructural and metabolic changes following stem cell transplantation in stroke-injured rat brains
- Pardes Habib, Neurosurgery, SOM
- PI: Gary K. Steinberg, Neurosurgery and Neurosciences, SOM
This project will explore the use of human neural stem cells (hNSCs) to treat chronic ischemic stroke in rats. Focusing on a specific MRI signal in the premotor cortex that correlates with clinical improvement, researchers aim to understand how hNSCs facilitate brain recovery. Advanced imaging techniques will be used to map microstructural and metabolic changes, potentially identifying new biomarkers and treatments for future clinical trials.
MRI evaluation of a novel murine model of RhoBTB2-related epileptic encephalopathy facilitating development of an antisense oligonucleotide therapeutic
Thomas LaRocca, Pediatrics, SOM
This project will develop a new mouse model to study a rare form of epilepsy linked to the RhoBTB2 gene, which currently has no effective treatments. Utilizing MRI, researchers will investigate how this genetic variant influences brain development and function. This team aims to use these insights to uncover structural relationships and neurodevelopmental impacts of the gene variant, potentially leading to novel treatment options for patients with this severe condition.
Assessment of MAP4K4 in regulating tumor cell invasion in a preclinical model of pediatric high-grade glioma
Laura Prolo, Neurosurgery, SHC, SOM
This project will study the role of MAP4K4, a critical kinase in signaling pathways, in the progression of pediatric high-grade gliomas (pHGGs)—aggressive brain tumors with few effective treatments. By employing advanced genetic editing tools like CRISPR and using MRI technology, researchers aim to understand how this protein impacts tumor growth and resistance in mouse models. The research will test whether inhibiting MAP4K4 reduces tumor invasion and improves survival, potentially identifying new targets for therapeutic intervention.
Investigating longitudinal white matter changes after juvenile stroke
- Elizabeth Mayne, Neurology, SOM
This project will investigate how strokes affect brain development in children differently than in adults, focusing on white matter, which facilitates brain communication. Preliminary data suggests that the response to stroke in young brains involves unique inflammatory processes that disrupt myelin formation, leading to cognitive challenges. By comparing juvenile and adult mice using advanced neuroimaging, this project aims to uncover age-specific responses and inform targeted therapies for young stroke survivors.
Mapping myelin plasticity in mouse models of generalized epilepsy
- Juliet Knowles, Neurology and Pediatrics, SOM
This project will explore myelin plasticity in mouse models to better understand epilepsy, a condition where brain networks change unfavorably, often leading to medication-resistant seizures and cognitive decline. By using MRI, the study aims to map how myelin—the protective coating on nerve cells that facilitates brain communication—is altered by seizures. The project will test whether these myelin changes can be reversed and will seek to understand the underlying mechanisms, with the ultimate goal of developing treatments that prevent worsening seizures and cognitive loss in children with epilepsy.
Watch a recording of the symposium here
Learn more about the Neuroscience Preclinical Imaging Laboratory (NPIL)