Project Summary
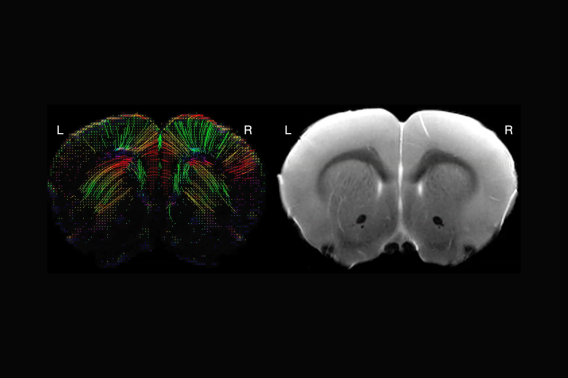
There are over 6000 rare diseases which together affect millions of Americans. Many ultra-rare diseases are ‘orphan’ diseases, without any viable treatment strategies, one of these being human RhoBTB2 variants associated with severe developmental epileptic encephalopathy (DEE64). Our translational laboratory is investigating the impact of the nano-rare RhoBTB2 p.R483H variant on brain structure and function that leads to impaired proteasomal degradation of the protein and aberrant accumulation within the cell. RhoBTB2 is an atypical Rho GTPase that may play a role in neurological function and dendritic development. We have identified an 8yo patient with RhoBTB2R483H/+ with severe progressive epilepsy that may be amenable to antisense oligonucleotide (ASO) therapeutics. We are currently designing an ASO for this patient as a novel treatment approach, and in parallel have generated a novel mouse model RhoBTB2R461H/+ that is orthologous to human RhoBTB2R483H/+ to gain better understanding of RhoBTB2 biology. In collaboration with the laboratory of Dr Juliet Knowles, mice were phenotyped using a battery of standardized neurodevelopmental assays and video EEG. Preliminary data suggests the RhoBTB2R461H/+ mouse exhibits significant growth restriction, grip strength, enhanced marble burying capacity, and spontaneous seizures as compared to littermate controls. These preliminary results provide evidence of RhoBTB2R461H/+ being a faithful in vivo rare disease model of DEE64. This project aims to characterize this novel mouse model of the RhoBTB2 p.R483H mutation utilizing MRI to identify structural relationships of the neurodevelopmental impact of RhoBTB2 variants.
Project Details
Funding Type:
Neuroimaging Pilot Grant
Award Year:
2024
Lead Researcher(s):