Seeking better treatments for preterm babies in the “second brain”
Preterm births have been rising in the United States since 2014, according to the Centers for Disease Control.
Fortunately, the survival rate for infants born before 37 weeks of pregnancy has been climbing, but caring for babies born at the extreme edge of viability (before about 22 weeks), is a daunting challenge for doctors because internal organs are still forming. To develop, preterm babies need milk, but due to limited gut motility – the movements that push food through the digestive tract, these babies often get severe intestinal obstructions or infections.
Researchers with Stanford’s Wu Tsai Neurosciences Institute aim to improve gut motility and health outcomes for preterm babies through foundational research on the nervous system of the gut, called the enteric nervous system (ENS). In a paper published on May 9, 2023 in Nature Communications, the team revealed how and when the neurons that control gut motility form and identified potential treatment avenues to improve outcomes for preterm babies.
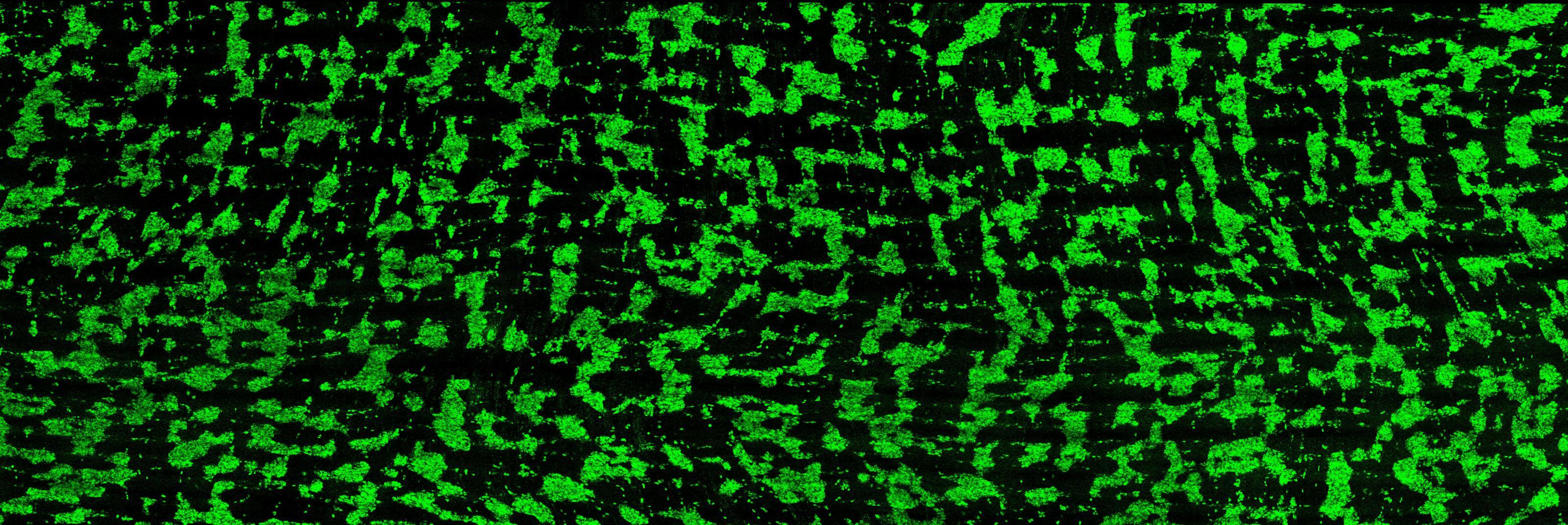
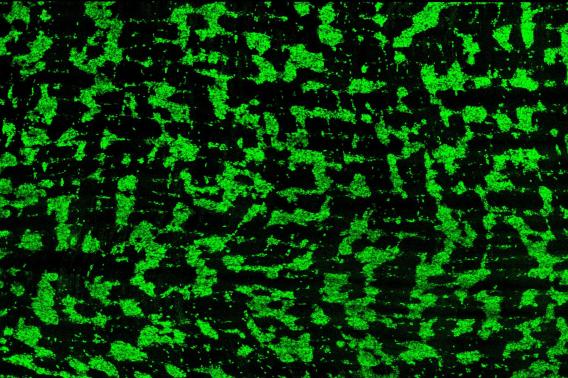
“We’ve now looked at both human and mouse intestines and found that the neurons that control motility organize themselves into a striped pattern,” said Julia Kaltschmidt, PhD, a faculty scholar at the Wu Tsai Neurosciences Institute and associate professor of neurosurgery at Stanford Medicine. “The emergence of these stripes coincides with the onset of gut motility in both species, which supports the hypothesis that these stripes are important for gut function.”
Learn more about the Second Brain
We spoke with Kaltschmidt on our podcast "From Our Neurons to Yours".
Listen here.
The ENS, which lines the walls of the gut and controls gut motility, is sometimes called “the second brain”. Comprised of some 500 million neurons and able to function independently of the central nervous system, the ENS also relies on many of the same neurotransmitters that regulate mood and cognitive function in the brain.
This complexity means that there are still large gaps in our understanding of the ENS, which is precisely what Lori Bowe Dershowitz, an MD, PhD candidate in the lab, set out to help remedy. In a previous study, Dershowitz dissected and visualized the structure of the embryonic mouse ENS and found that neurons in the intestines developed in a striped pattern. She wondered if human intestines might have the same neuronal organization.

“When we looked at human fetal intestines, we found the same striping pattern,” Dershowitz said. “This allowed us to make new conclusions about the structure of the enteric nervous system.”
One structure newly identified by the team was a complex neuronal grid overlaying the neuronal stripes. The function and purpose of this grid is yet to be discovered, but the researchers are hopeful that their understanding of neuronal stripes in the human gut will lead to new treatments to help preterm babies process food.
“One of the major challenges with preterm infants is that they can’t receive intravenous nutrition alone. If the intestines don’t get milk, they’ll atrophy,” said senior co-author Anca M. Pasca, MD, an assistant professor of pediatrics at Stanford Medicine and a Wu Tsai Neuroscience Institute affiliate. “The intestines of preterm infants have minimal motility, so their stool can’t be eliminated. This can cause obstructions and perforations that require surgery. We desperately need a better understanding of the ENS to help us find treatments that improve motility and milk tolerance.”
Lack of motility occurs because the ENS is still incomplete in preterm infants. In a fully formed digestive system, material moves through the intestines via a series of coordinated contractions toward the colon. In studying fetal intestines, the researchers found that, at around 18 weeks, the neurons in the intestines were active, but not synchronized. Intestinal contractions were sporadic and moved in both directions – toward and away from the colon. They found that the earliest occurrence of synchronized contractions was at 21 or 22 weeks.
The team suspects that uncoordinated gut movements in preterm infants might be due to uneven development of neurons. Those that cause the intestines to relax emerge first and outnumber neurons that cause contraction until about 21 weeks.
“Seeing how this ratio of neurons changes over time will help us find pharmacological treatments to increase motility in preterm infants. We know that drugs used to increase motility in adults don’t work in the preterm infants, and that may be because the composition of enteric neurons is very different,” said Dershowitz.
In newer still unpublished experiments, the lab is finding evidence that increased motility can be achieved in embryonic mice with specific drugs that restrict certain neurons. This allows the less-developed contraction-associated neurons to move matter through the intestines. The team is hopeful that their findings will help identify drugs that can be used to increase gut motility for preterm babies, potentially optimizing their growth and increasing their survival rates.