Neuroscience sheds light on childhood gut disorders
Wu Tsai Neuro Faculty Scholar Julia Kaltschmidt and MD/PhD candidate Lori Dershowitz propose a novel theory that could transform our understanding of pediatric gastrointestinal (GI) motility disorders.
Their proposal was published March 11, 2024, in a Perspective article in the journal Cellular and Molecular Gastroenterology and Hepatology.
The enteric nervous system (ENS), sometimes referred to as the "second brain," is a complex network of about 500 million neurons that governs the function of the GI tract. It is the largest branch of the peripheral nervous system and is as diverse as the central nervous system.
“ENS function is vital to life,” Dershowitz explained. “From the moment of birth, defects in GI motility can be life-threatening. Newborn babies can’t leave the hospital until they’ve proved they can poop! However, the ENS is still poorly understood and we have no drugs that directly target ENS function.”
The relationship between the organization of the ENS and the drivers of GI diseases has remained a mystery. This is particularly relevant as two-thirds of American adults report suffering from GI distress, and childhood GI motility disorders such as Hirschsprung’s disease (HSCR), pediatric intestinal pseudo-obstruction (PIPO), and intestinal neuronal dysplasia type B (INDB) are linked to structural deficits in the ENS.
“As a medical student, I have taken care of many patients with GI dysfunction of unknown etiology. The combination of GI dysmotility and a lack of explanation causes a lot of suffering for patients,” Dershowitz said.
“I hope that physicians and scientists will think more critically about how the ENS may be underlying dysfunction in GI disorders and patient complaints that are often dismissed.”
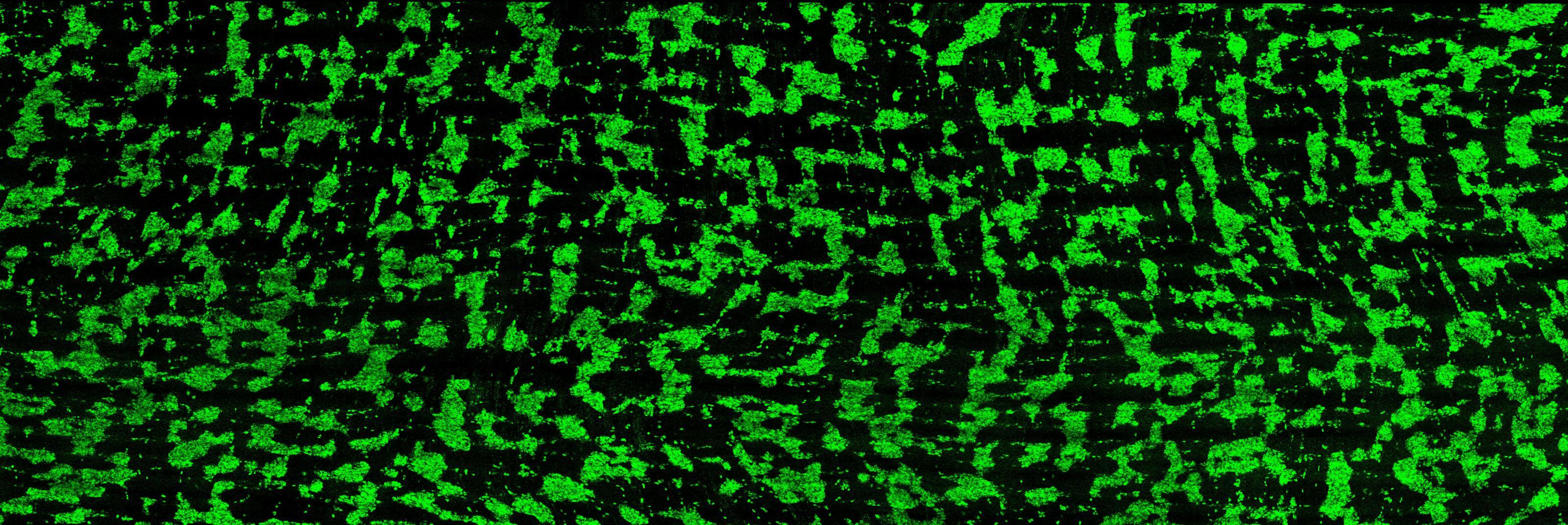
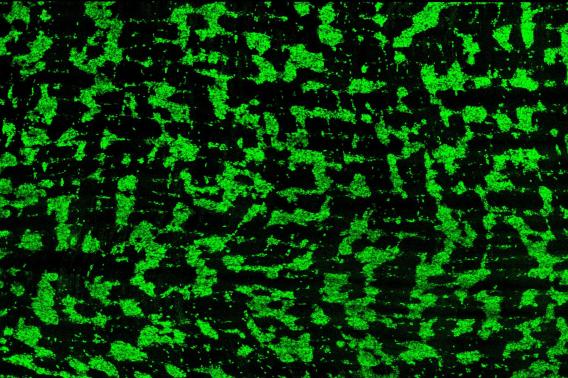
Dershowitz and Kaltschmidt, an associate professor of neurosurgery at Stanford Medicine, recently discovered that around the time of birth, the ENS organizes itself into circumferential stripes along the length of the intestine, a pattern that they observed in both mice and humans. They hypothesize this patterning is needed for the rhythmic peristalsis that keeps the gut’s contents moving, allowing infants to begin digesting food independently after birth.
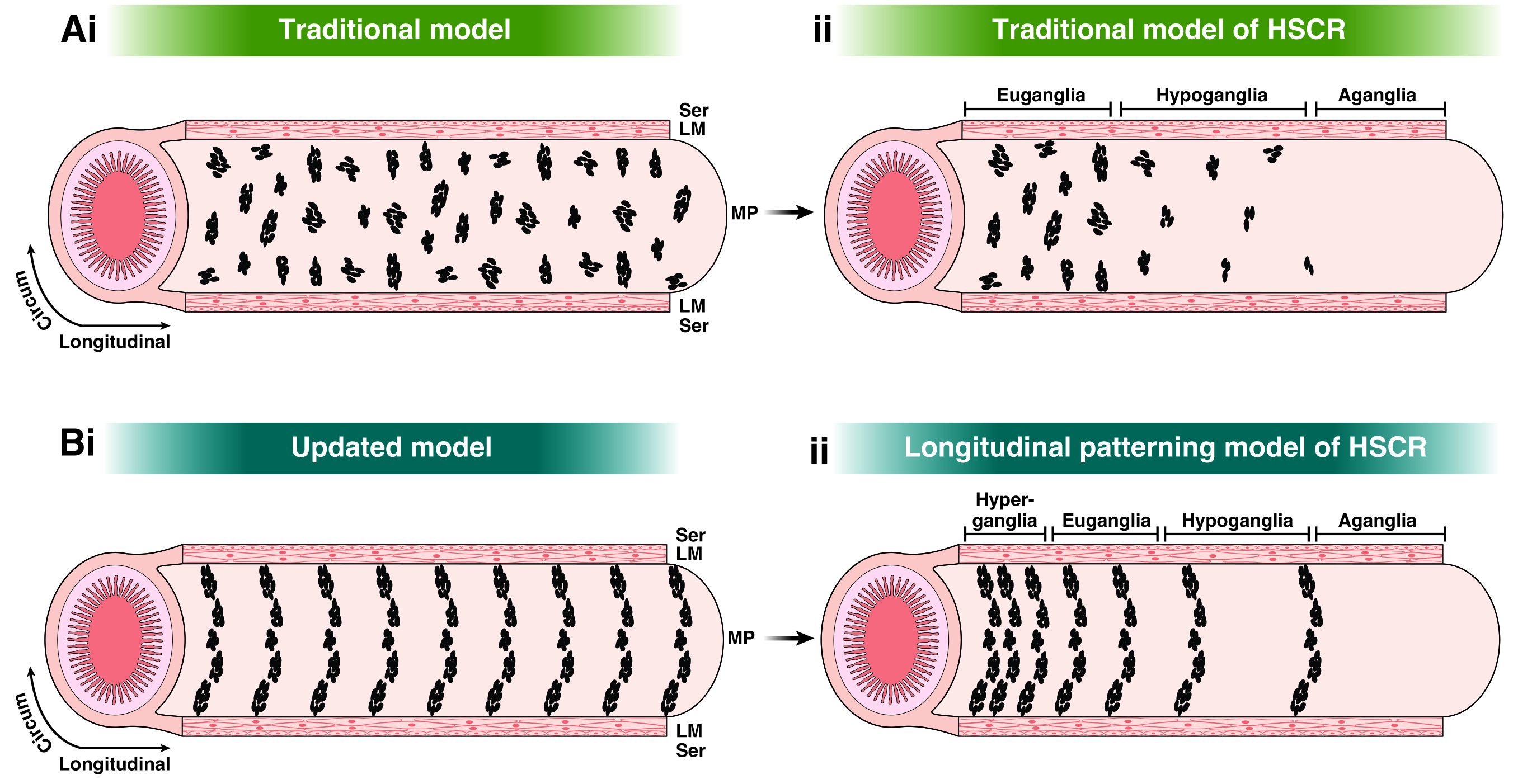
In their new Perspective article, Dershowitz and Kaltschmidt propose that pediatric GI motility disorders may arise from defects in the patterning of enteric neuron stripes along the longitudinal axis of the intestine. They suggest that changes in the regularity of these stripes could explain the diverse structural pathologies observed in these disorders.
For instance, increased distances between ENS stripes could result in low enteric neuron density, a characteristic of HSCR, while decreased distances between stripes could pack enteric neurons too closely, a feature of PIPO and INDB. The absence of stripes could explain the occurrence of skip lesions, a poorly understood phenomenon observed in HSCR.
The researchers argue that the current methods of assessing intestinal biopsies may underrepresent structural defects in pediatric GI motility disorders. They recommend that future studies examine larger tissue samples or utilize noninvasive imaging techniques to assess any changes to striped patterning.
“We are excited to further explore the organization and function of the ENS in health and disease,” said Kaltschmidt. “Our goal is to generate a ’map’ or ‘blueprint’ of ENS cytoarchitecture and explore the molecular determinants of stripe development, which we hope will facilitate our understanding of GI disorders that do not as of yet have a known enteric neural correlate.”
A better understanding of the biological origins of pediatric GI motility disorders could lead to improved diagnostic methods and treatments, Dershowitz added, potentially transforming the lives of children and families affected by these conditions.
“I am excited to explore mechanisms that may underlie the development of stripes in the ENS, and I’m curious whether these mechanisms can be implicated in human disease,” she said. “I hope that physicians and scientists will think more critically about how the ENS may be underlying dysfunction in GI disorders and patient complaints that are often dismissed.”