Synthetic neuroscience grants promote transformative brain tech

The Wu Tsai Neurosciences Institute, Sarafan ChEM-H, and Stanford Bio-X have awarded $1.24 million in grants to five innovative, interdisciplinary, and collaborative research projects at the intersection of neuroscience and synthetic biology.
The emerging field of synthetic neuroscience aims to leverage the precision tools of synthetic biology — like gene editing, protein engineering, and the design of biological circuits — to manipulate and understand neural systems at unprecedented levels. By creating custom-made biological components and integrating them with neural networks, synthetic neuroscience offers new ways to explore brain function, develop novel therapies for neurological disorders, and even design biohybrid systems that could one day allow brains to interface seamlessly with technology.
"The ongoing revolution in synthetic biology is allowing us to create powerful new molecular tools for biological science and clinical translation,” said Kang Shen, Vincent V.C. Woo Director of the Wu Tsai Neurosciences Institute. “With these awards, we wanted to bring the Stanford neuroscience community together to capitalize on this pivotal moment, focusing the power of cutting-edge synthetic biology on advancing our understanding of the nervous system — and its potential to promote human health and wellbeing."
Institute-led community-building sets stage for transformative partnerships
“There’s been a lot of conversation about synthetic biology around campus, but neuroscience hasn’t traditionally been a central player in those discussions,” said Jill Wentzell, Wu Tsai Neurosciences Institute Executive Director. “The intersection of these two fields is of great interest to Wu Tsai Neuro, Sarafan ChEM-H, and Bio-X, making it an ideal opportunity for the three institutes to collaborate, strengthen the community across the life sciences, and synergize our interdisciplinary efforts.”
The institutes requested proposals by teams of two or more Stanford scientists for groundbreaking research projects to address important research problems in synthetic neuroscience that could not be pursued without combined interdisciplinary expertise. Each selected project was awarded $250,000 over two years to pursue this research.
A newly funded project to engineer protein-based tools to study the effects of psychedelics on the brain epitomizes this cross-disciplinary orientation. “This partnership … connects deep expertise in synthetic chemistry with systems-level psychedelic neuroscience,” said Boris Heifets, an associate professor of anesthesiology who received the award with Justin Du Bois in the Department of Chemistry. “Together, we plan to develop a method that may give us insight into the basic cellular mechanisms that account for the experiential and possible therapeutic effects of psychedelics.”
The awards follow a series of Synthetic Neuroscience forums hosted by the three institutes last winter that engaged hundreds of faculty and trainees in wide-ranging conversations about research opportunities in this emerging interdisciplinary field.
Institute affiliate Bradley Zuchero, an assistant professor of neurosurgery, and Noa Katz, a postdoctoral scholar in chemical engineering, say their newly funded project was “sparked” by a conversation they struck up at one of the Institute-sponsored forums. Now they are working together to design programmable molecular circuits within brain cells to study the contributions of myelin, which makes up the brain’s white matter, to neurodegenerative disorders.
“We realized then that we had shared interests in myelin and synthetic biology, and that by combining these interests we could make brand new tools that will open up many exciting new research directions for us and the field,” Zuchero said.
“This is an amazing, one-of-a-kind opportunity for me as a postdoctoral scholar,” Katz added. “I am excited to work together with Professor Zuchero in combining our expertise to create a new framework for tackling major conceptual challenges in myelin biology.”
Funded Projects
Defining the temporal and spatial CSF secretome by TurboID labeling
- Ryann Fame, assistant professor, Neurosurgery
- Jonathan Long, associate professor, Pathology
The cerebrospinal fluid (CSF) that surrounds the brain and spinal cord also influences the development, maturation, and aging of the nervous system in ways that are not fully understood. This team will leverage innovative bioengineering tools to better understand the myriad proteins found in CSF and how they arise from various sources across the body. The proposed research will employ TurboID, a synthetically engineered enzyme, to selectively label CSF proteins secreted from particular source tissues. By tracking the sources and development of these proteins, this research will offer insight into the roles the CSF plays in health, aging, and disease.
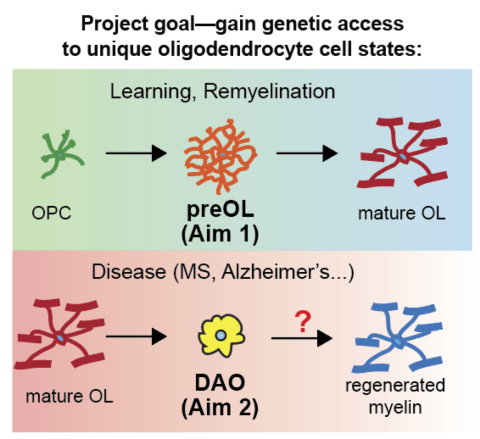
First-in-class RNA sensors for studying myelin dynamics and disease
- Bradley Zuchero, assistant professor, Neurosurgery
- Noa Katz, postdoctoral scholar, Chemical Engineering
This team leverages state-of-the-art synthetic biology tools to understand how oligodendrocytes—a type of non-neuronal cell that forms the critical myelin insulation around nerve fibers in the brain—contribute to Alzheimer’s disease and other demyelinating disorders. The proposed research will use RNA sensors, a cutting-edge tool in synthetic biology for probing complex molecular pathways within cells and for creating “smart” molecular circuits within cells for use in gene therapy and other approaches. The team proposes creating two synthetic molecular circuits within oligodendrocytes: one that can activate myelin repair and survival when it senses optimal conditions for regrowth, and a second that senses disease-associated states within the cell to help researchers understand and reverse their contribution to the disease.
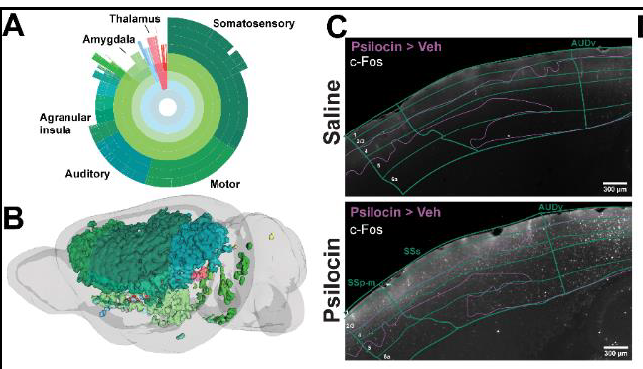
Traceless fluorescent labeling of endogenous 5-HT2A receptors
- Boris Heifets, associate professor, Anesthesiology
- Justin Du Bois, professor, Chemistry
Treatments for mental health conditions such as depression have remained stagnant for decades. Psilocybin and other psychedelic drugs represent a beacon of hope, showing great promise to advance psychiatric medicine. Psychedelics profoundly alter human consciousness through activation of 5-HT2A receptor proteins in the brain, and these effects are correlated with therapeutic outcomes. While extensive resources are being invested to understand psychedelic mechanisms of action, currently available tools are insufficient to visualize native 5-HT2A receptors in the living brain. This research proposal aims to develop a new small molecule probe that permanently illuminates 5-HT2A receptors in living cells and brain tissue without modifying the receptor function or expression. We anticipate this cutting-edge tool to give researchers a new way to study the therapeutic and physiological role of 5-HT2A receptors, empowering innovation and ultimately leading to more effective treatments for mental health disorders.
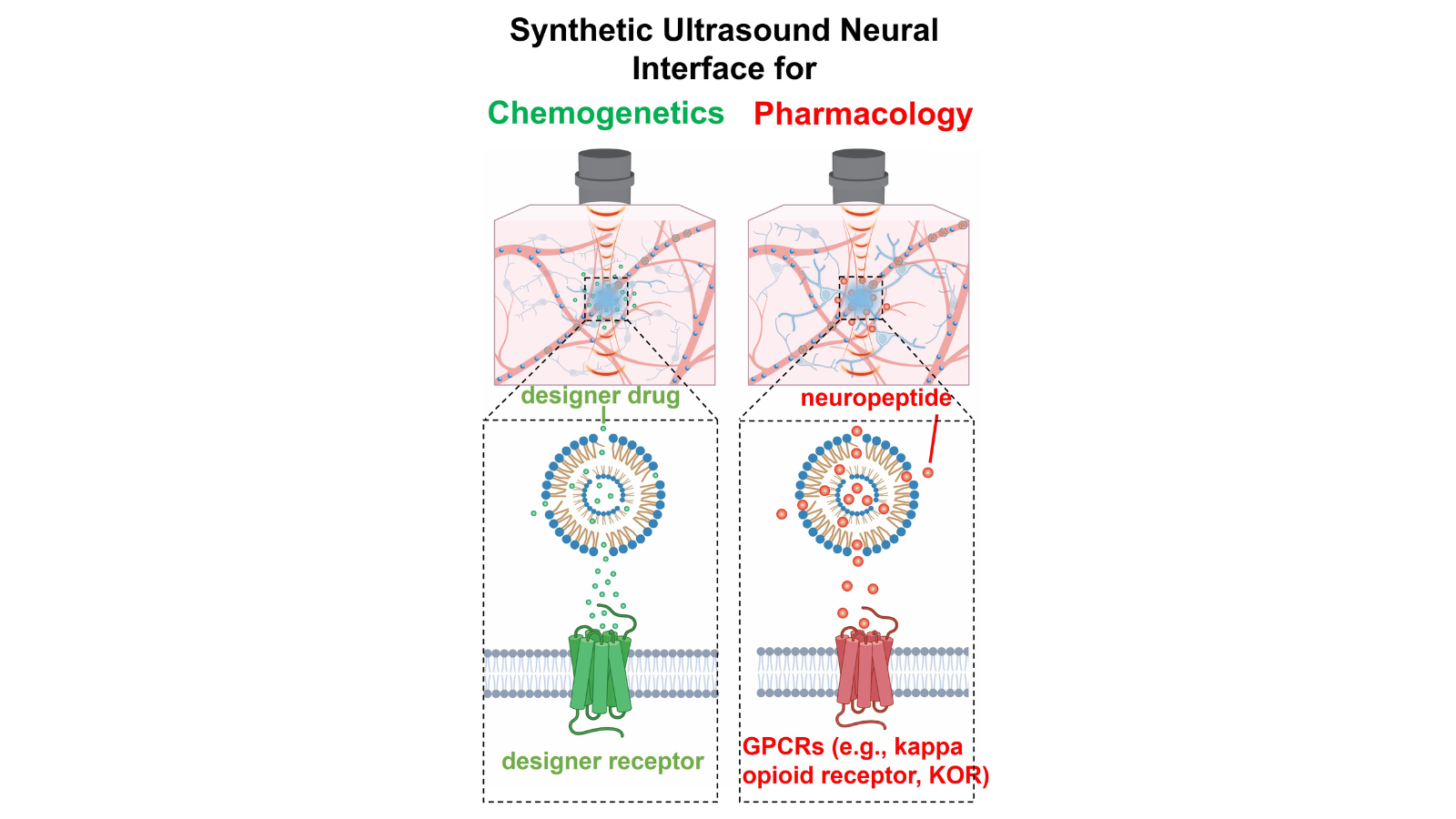
A synthetic ultrasound neural interface for non-invasive and spatiotemporally precise chemogenetic and pharmacological neuromodulation
- Guosong Hong, Wu Tsai Neurosciences Institute Faculty Scholar, assistant professor, Materials Science and Engineering
- Jun Ding, associate professor, Neurosurgery & Neurology and Neurological Sciences
Controlling brain activity using chemicals and drugs is instrumental in neuroscience research. Neuroscientists rely heavily on drugs and genetically engineered chemical sensors to study and manipulate brain circuits with neuron-type specificity in behaving animals. However, current delivery methods for these compounds are imprecise. The proposed research will develop a synthetic neural interface to allow for more controlled chemical and drug release by using ultrasound to precisely penetrate neural tissue. This technology will allow researchers to noninvasively dissect functional neural circuitry with improved spatial and temporal resolution to control brain activity more precisely, and to enable manipulation of multiple regions simultaneously. The team predicts that this technology will offer widespread applications in precise delivery of molecules across the human brain.
Genetically-encoded voltage integrators for stable tagging of activated or inhibited neural ensembles in vivo
- Xiaoke Chen, associate professor, Biology
- Shang Jui Tsai, postdoctoral scholar, Genetics
A major goal in systems neuroscience is to discover how patterns of activity in neural circuits produce and regulate behavior. Current techniques let scientists visualize activated neurons, but cannot detect inhibited neurons, which are equally important for producing and understanding behavior. This team will use synthetic biology to address this gap. By applying tools of protein engineering and directed evolution to natural voltage-sensing domains, the proposed research will develop first-in-class genetically encoded voltage integrators (GEVIns) capable of sensing and responding to both activation and inhibition of neurons. The researchers aim to apply GEVIns to identify neural subpopulations in the spinal cord that are inhibited in chronic pain.