Ultrasound for the brain
Esther Landhuis
An ultrasound treatment for a movement disorder called essential tremor has calmed a man’s shaking hands for the first time in ten years.Credit: Canadian Press/REX/Shutterstock
A man comes into the clinic, hands trembling. He can barely write or hold a cup of water. He straps on a specialized head device, lies back and slides into a magnetic resonance imaging (MRI) scanner. Across the room, a physician pushes a button — and the shaking stops. Handed a piece of paper, the man signs his name legibly, hands steady.
This kind of transformation is not wishful thinking. Videos documenting such interventions are readily available online. At their heart is a newly approved technology that uses MRI to aim ultrasonic waves — best known for use in prenatal monitoring — at specific areas of the brain. “We can focus the ultrasound through the skull to a part of the thalamus about the size of a grain of rice,” says W. Jamie Tyler, a neuroscientist who studies non-invasive brain stimulation at Arizona State University in Tempe. In that scenario, ultrasound heats and kills neurons in the thalamus, the brain area thought to give rise to a movement disorder called essential tremor, which affects millions of people worldwide. Last year, the US Food and Drug Administration (FDA) approved focused ultrasound thalamotomy as a treatment for people with essential tremor who haven’t responded to medication.
Today, some scientists are setting their sights on another frontier. By influencing the brain more subtly — zapping small groups of neurons enough to boost or suppress their activity without killing them outright — ultrasound could potentially treat other movement disorders, as well as depression, anxiety and a host of intractable neuropsychiatric disorders, as easily and painlessly as the procedure that calms shaking hands, says Shy Shoham, a biomedical engineer from the Technion–Israel Institute of Technology in Haifa who is starting a lab at New York University Langone Medical Center.
The emerging technology, called focused-ultrasound neuromodulation, makes use of energies at least an order of magnitude lower than those used for treating tremor. Instead of blasting and killing brain cells, Shoham says, “you basically dial down the system”.
To some extent, other non-invasive techniques can already do this using magnetic fields or direct electric currents. Transcranial magnetic stimulation (TMS) has been approved in the United States, the European Union and elsewhere to treat people with depression who have not benefited from antidepressant medication. Transcranial direct-current stimulation (tDCS) has won approval in the EU for treating depression and pain disorders and is used widely on an experimental basis.
But the effects of these methods on neurons are varied, hard to measure and limited in penetration and focus. Electric and magnetic signals strongly influence cells near the brain’s surface but fade just a centimetre or two deeper, producing a gradient effect. Ultrasound, by contrast, can be targeted with high precision. “What makes it an interesting tool is its potential to affect deep regions of the brain selectively, which is not possible with current electromagnetic approaches,” says Michael Nitsche, a brain-stimulation pioneer at the Leibniz Research Centre for Working Environment and Human Factors in Dortmund, Germany. “This could be a breakthrough.”
Researchers and clinicians are taking notice. Some 280 publications on “ultrasound neuromodulation” have been indexed in the PubMed database since 2007 — a 14-fold increase over the previous decade. And at least one funding call, from the US Defense Advanced Research Projects Agency, has been issued specifically for the development of ultrasound devices for imaging and neuromodulation. Yet important questions remain, and clinical use is years away. Chief among those questions is what ultrasound actually does to neurons inside the brain. “There’s a whole lot of something going on, but we’re not quite sure what that something is,” says Raag Airan, a neuroradiologist at Stanford University in California.
In the meantime, the technology could prove useful for mapping neural circuits in the brain, and for answering other clinical and basic-research questions.
Early work
Airan’s foray into ultrasonic brain stimulation began in 2014, with some old equipment that he found collecting dust in the hospital basement at Johns Hopkins Hospital in Baltimore, Maryland. At the time, Airan was doing his radiology residency. Digging into the literature, he learnt that more than 80 years earlier, researchers had placed a frog leg and heart into a salt solution and seen the muscles twitch as they passed ultrasound waves through the bath. It was one of the first demonstrations of ultrasound’s effect on nerve-cell activity1. In 1958, experiments in cats showed that ultrasound could influence the animals’ neurological responses to light2.
Half a century later, work by Tyler and colleagues provided clues to the mechanism underlying those experiments. Working with cultured mouse hippocampal slices, the team found that ultrasound triggers nerve impulses by activating voltage-gated sodium and calcium channels3. In 2010, the researchers showed that ultrasound could remotely stimulate brain cells in the motor cortices of anaesthetized mice4. And a few years later, researchers at INSERM — France’s biomedical-research agency based in Paris — replicated the effect in brain areas that control eye movements in conscious monkeys5.
Eventually, in 2014, Tyler’s group advanced the technique into humans, stimulating the somatosensory cortex, a brain area that processes tactile information6. A team led by Seung-Schik Yoo of the Catholic University of Korea in Incheon extended that work to the primary visual cortex, which controls eyesight, in 20167 .
The monkey and human studies highlighted two key benefits of the technology. First, they demonstrated that brain areas that could be activated using other non-invasive techniques could also be stimulated more precisely with ultrasound, Nitsche notes. Second, whereas earlier research had involved anaesthetized animals, the more recent studies showed that ultrasound could drive brain activity and corresponding complex behaviours in primates that were fully awake — and could potentially reach much deeper areas of the brain. “That was a big deal,” Shoham says.
Poring over these papers, Airan was intrigued. But, from a clinical standpoint, he found the reported effects to be small. The human studies showed that “you can get an effect”, Airan says. But as a physician, “I don’t want just an effect. I want an effect that’s going to definitely do whatever the prescribed thing is, each and every time I apply it.”
To put things in perspective, when a TMS magnetic transducer is placed over the brain’s motor area, a person’s fingers can be made to snap up and twitch. If ultrasound were to influence nerve cells with equivalent power, Airan reasoned, it should produce a similarly dramatic behavioural response. But in the somatosensory experiments, even the largest effects were subtle, instead amounting to improved performance on a nerve-sensitivity test called two-point discrimination, which gauges how well a person can distinguish nearby pin pricks as two points instead of one.
Despite superior precision and penetration, ultrasound’s effects in the brain tend to be weaker than those achieved using electromagnetic stimulation, and harder to study. Because ultrasound is a pressure wave, it’s thought to create small vibrations that could throw off electrode recordings in electrophysiology studies of cells and brain slices. This is less of a concern with other brain-stimulation approaches, because they transmit electromagnetic waves. In addition, ultrasound’s effects in rodents are particularly sensitive to the level of anaesthetic administered.
Using the abandoned Hopkins equipment, Airan tried some experiments in rats, zapping their brains and looking for the expected tail flick. He saw the response barely one-third of the time. So, to obtain a more robust and reliable effect, Airan borrowed a page from oncology. Clinical oncologists can deliver medicines to tumours by hiding the drugs inside nanoparticles that recognize specific molecules on the surface of cancer cells. Similarly, Airan’s team first loads drugs into nanoparticles, then injects them into rodents. After that, the researchers apply ultrasonic waves to the brain, which causes the nanoparticles’ outer shells to vaporize and releases the drug into surrounding tissue (see ‘Ultrasonic drug delivery’).
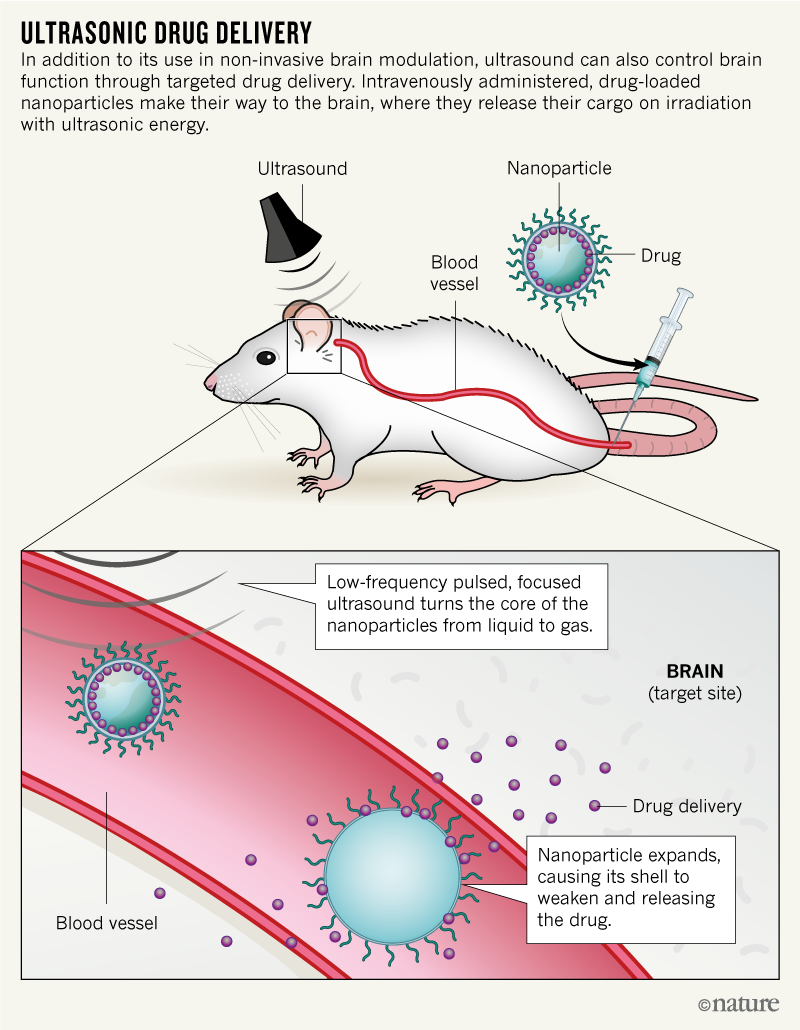
“We know how a bunch of drugs work in the brain,” Airan explains. If ultrasound could be used to “put the drug in one part of the brain at a particular time, then I could get the precision of focusing ultrasound deeply in the brain and know that whatever effect [shows up] is due to that particular drug being there.”
Nanoparticle success
In a study published earlier this year8, Airan used ultrasound to precisely trigger the release of propofol — an anaesthetic that slows the activity of the brain and nervous system — in the brain of a rat model for seizures. “We were able to silence the seizures totally,” Airan says. And “if you’re able to knock out seizures, you can definitely knock out regular brain activity”.
In the hope of speeding the path to human testing, the team submitted an application in September to the US National Institutes of Health Nanotechnology Characterization Laboratory in Frederick, Maryland. Should the lab accept the proposal, it will perform toxicity studies on the Stanford team’s nanoparticles. Depending on how those go, phase I testing could begin in a year or two, Airan says.
Nitsche is excited about the findings so far. If the nanoparticle approach is shown to work in people, he says, its precision could “enable targeted application of drugs regionally in the brain, which could be promising for many brain diseases”, and reduce side effects, as well.
But precisely how the technique works remains unclear. At high intensities, such as those used to relieve essential tremor, ultrasound’s effects are largely thermal: the tissue heats up and cells die. The effects of low-intensity ultrasound, however, are more likely to be mechanical, and tougher to tease apart. One way to look at it, Airan says, is that cells experience something akin to what is felt by a person standing near a strong bass speaker. “You can literally feel the boom,” he says. In that scenario, the ultrasound acts like a pressure wave — it pushes on the tissue, creating vibrations that cause cell membranes to weaken. That, in turn, could affect nerve-cell firing, because certain ion channels are mechanically sensitive and so respond to stretches and strains within the membrane. Another possibility is that ultrasound creates positive or negative pressure that causes individual cells to compress or expand. “The short answer so far: it’s complicated,” Airan says. A colleague of his also at Stanford, Kim Butts Pauly, is working to untangle the problem by determining which cell types in mice respond to these effects, and how those effects then translate into changes in neural activity.
Buying and building systems
Teasing the mechanistic complexities apart won’t be easy — or inexpensive. The clinical-grade system that received FDA approval in 2016 for treating difficult cases of essential tremor, made by Israel-based Insightec, costs between US$1.8 million and $2.8 million. Even preclinical devices for small-animal studies can run to a few hundred thousand dollars.
Certain customizations can bring costs down — and such changes are often necessary, given that most focused-ultrasound systems are set up for cell ablation. “That’s why for neuromodulation experiments, we end up putting our own stuff together,” Airan says.
While researching ultrasound systems last year, Airan realized that his mouse experiments didn’t need certain costly bells and whistles, such as real-time magnetic-resonance guidance. He purchased a base model from the French medical-device company Image Guided Therapy, and worked with the company to design a more barebones device, for around $60,000.
Other researchers create their own systems instead. Biomedical engineers Charles Caskey and Will Grissom at the Vanderbilt University School of Medicine in Nashville, Tennessee used focused ultrasound to induce anti-tumor immune responses in mice. Drawing from principles described in a 2011 protocol by Tyler and colleagues9, they cobbled together a small-animal ultrasound device using a commercially available transducer, amplifier and waveform generator, and hooked it up to a delivery table that they had constructed specially to slide inside an MRI scanner. Finally, they wrote software to integrate the ultrasound system with real-time magnetic-resonance images processed on a lab computer.
The total cost of parts came in at less than $25,000. “It was the only way we could get [the project] off the ground with the budget we had,” Caskey says. His team published the procedure last year in the Journal of Therapeutic Ultrasound10, and made assembly instructions and software available on the software-collaboration platform GitHub. A team led by biomedical engineers Weibao Qiu and Hairong Zheng at the Shenzhen Institutes of Advanced Technology in China slashed the cost by another order of magnitude, by building a system from base electrical parts rather than pre-assembled components11.
Although the Vanderbilt team’s ultrasound system was originally designed for other purposes, the team has since integrated the device with a strong (7-tesla) MRI magnet to conduct neuromodulation studies in the somatosensory cortices of non-human primates. “We’re stimulating that region with ultrasound, and imaging the functional activity that occurs in response,” Caskey explains. Similar experiments could use ultrasound as a tool for dissecting neural circuits that are thought to underlie schizophrenia, depression and other human brain disorders. By better understanding these circuits and working out how to modulate them, he says, researchers hope one day to help people with neuropsychiatric conditions by repairing or resetting the relevant neural pathways.
Ultrasound could also be used as an investigative tool in basic research. To address some questions, neuroscientists might chemically or otherwise modify how the brain is working. Or they could use ultrasound to non-invasively stimulate or inhibit neural circuits at a finer level — perhaps in only one region — to probe its connectivity with other brain regions, Caskey says. In the clinical realm, ultrasound could one day reduce some of the trial and error involved in placing electrodes for deep brain stimulation — an FDA-approved surgical treatment for essential tremor and Parkinson’s disease.
Although research into the mechanisms and engineering of ultrasonic neuromodulation continues apace, the field’s future, Tyler says, depends on the development of commercial systems designed specifically for this work. Homegrown systems tend to be harder to operate and maintain, he explains. “I cannot tell you how many hack-job microscopes I have seen abandoned after the one postdoc in the world who knew how to operate the open-source code and hardware left a lab.”
Ultrasound advocates are optimistic that such hardware is coming — and with it, a transformation in neuromedicine. “From a physics point of view, the potential advantages, especially for deep brain areas, are huge. There’s no question,” Shoham says. “But there are all kinds of practical issues that will need to be clarified.”