Alzheimer’s risk gene tied to fatty blobs in brain’s immune cells

Recent research identifies a significant link between the APOE4/4 gene, a known genetic risk factor for Alzheimer's, and the unusual buildup of fatty lipid droplets in microglia, the brain's immune cells.
This discovery suggests new directions for Alzheimer's treatment. Published in Nature on March 13, 2024, the study was led by Michael Haney and Róbert Pálovics and overseen by Tony Wyss-Coray, D.H. Chen Professor of Neurology and Director of the Knight Initiative for Brain Resilience at Stanford’s Wu Tsai Neurosciences Institute.
The team found that the Aβ protein, known for forming plaques in Alzheimer's patients' brains, prompts microglia to produce and store excess fats in droplets, a process especially common in those with the APOE4/4 gene.
These lipid-laden cells then release substances that harm brain cells and lead to the production of phosphorylated Tau, which forms the protein “tangles” that often accompany neurodegeneration in the brains of Alzheimer’s patients.
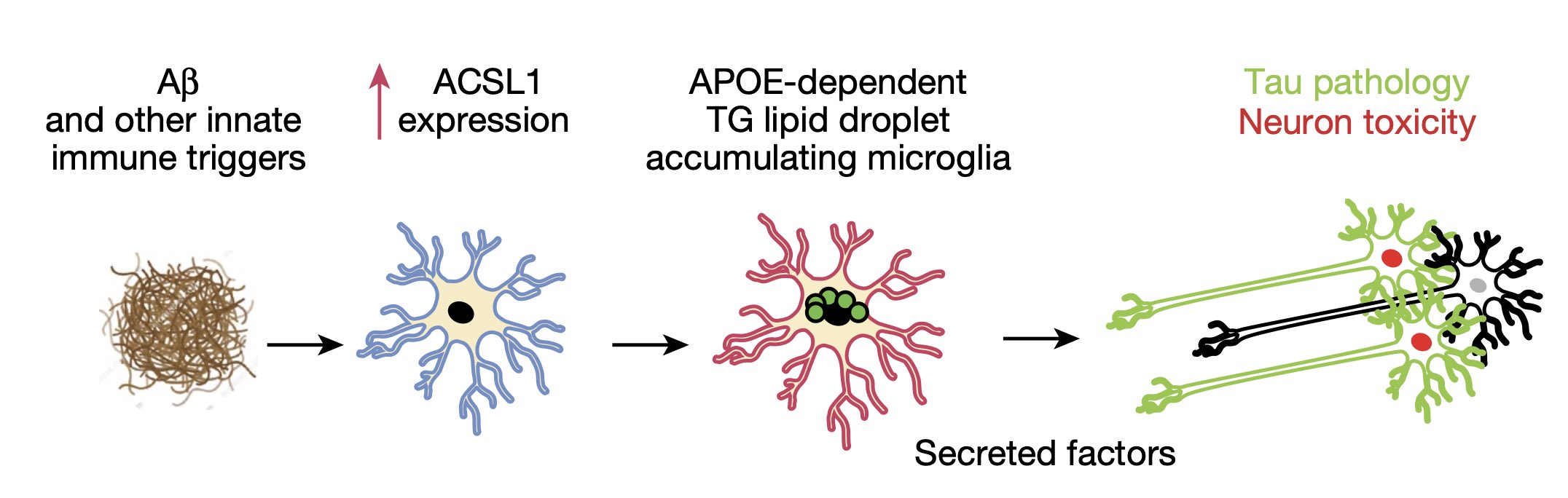
Schematic of the proposed role of LD + microglia in neurodegeneration. *P < 0.01, **P < 0.001,****P < 0.0001. Image credit: Wyss-Coray Lab.
This research highlights the role of fat metabolism in microglia, particularly in individuals with the APOE4/4 variant, in the progression of Alzheimer’s disease.
It also draws potentially informative biological links between the disorder’s classical pathology (amyloid plaques and tau tangles) and more recent evidence of the important role of lipid-related genes such as APOE and inflammatory microglia as drivers of neurodegeneration.
“We had previously characterized lipid droplets in mouse microglia and showed that they can be induced by classic triggers of the innate immune system — and that genes linked to neurodegeneration could regulate lipid droplet development,” Wyss-Coray said. “Since Abeta is recognized as an innate immune trigger by microglia, it is intriguing to speculate that lipid droplets may be part of a conserved yet maladapted response in the brain. It will be exciting to determine precisely what the neurotoxic factors are that these lipid droplet–containing microglia produce.”
The research raises the potential for targeting these processes as a new target for Alzheimer’s therapies, the authors say.
If scientists can figure out how to stop these harmful fats from accumulating or being transferred to microglia, especially in people with the APOE4/4 gene, they might be able to slow down or prevent the disease.
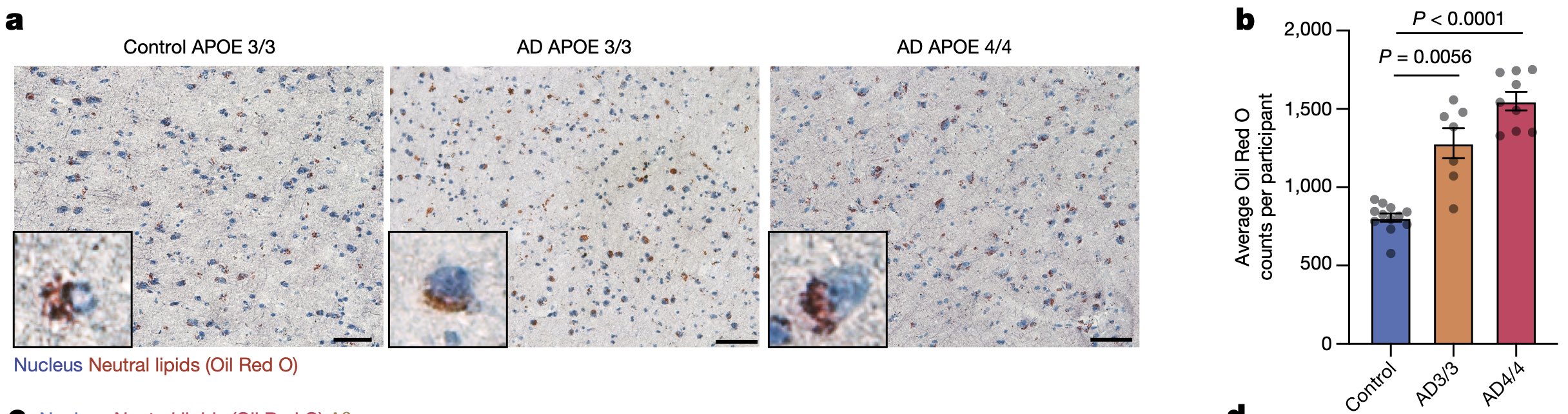
Representative OilRed O staining image for control, AD-APOE3/3, and AD-APOE4/4 human frontal cortex. Neutral lipids stained with Oil Red O (red) and nuclei stained with haematoxylin (blue). Scale bars, 50 μm. Image credit: Wyss-Coray Lab.


