Knight-funded research uncovers gene mutations that may prevent Alzheimer’s Disease
One in every four people who will read this article has a time bomb lurking in their DNA. Just one of those time bombs—known in scientific parlance as the gene variant APOE4—increases someone’s odds of developing Alzheimer’s disease threefold. Unless they are extremely lucky, that is: new research supported by the Knight Initiative for Brain Resilience suggests that some severe mutations can defuse the APOE4 time bomb.
A team led by physician-scientist Michael Greicius, the Iqbal Farrukh and Asad Jamal Professor in the Department of Neurology, scoured a large-scale genetic database for individuals with severe mutations to the APOE gene. Based on a small sample of seven individuals, the team was able to conclude that disabling APOE4 is likely to protect against the development of Alzheimer’s disease. They published their results in the leading journal Neuron in February.
It might seem obvious that inactivating a disease-associated gene variant would reduce its ability to do damage. In fact, the result was far from a foregone conclusion. Many harmful gene variants drive disease by producing disabled proteins that fail to do their necessary jobs, like an injured border collie who is too feeble to shepherd its flock. But others generate overpowered mutants that actively wreak havoc on the body: a rabid dog biting and infecting the sheep under its care. The distinction is critical to designing treatments: The first class needs to be healed or replaced; the second needs to be put down.
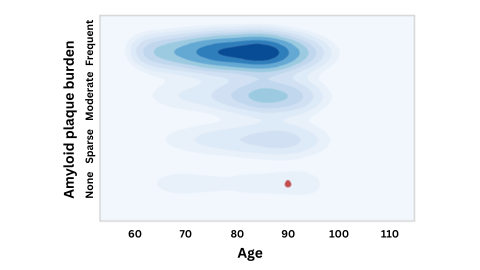
A figure emphasizing that an APOE-4 carrier with virtually no amyloid buildup at age 90 (red dot) is an extreme outlier compared to others with this gene variant. This suggests the APOE gene may have been "defused" by another, protective mutation. Image by Michael Greicius.
These questions have taken on new urgency with the rapid advancement of new genetic therapies — such as so-called “antisense therapies” recently approved by the FDA, in which short segments of genetic material called “antisense oligonucleotides” can be deployed to artificially deactivate genes. The antisense therapy Nusinersen, for example, is the first-ever drug approved to treat spinal muscular atrophy, a devastating disease that often kills those affected before their fourth birthday. But such treatments must be deployed judiciously: fully blocking a weakened gene would only worsen the problem.
Based on the new findings, Greicius and his team concluded that the APOE4 mutation seems to be of the second variety, since individuals with fully disabled copies appear to be protected from Alzheimer’s. These results, Greicius said, give him confidence that disabling APOE4 might be a promising path forward for fighting Alzheimer’s disease. “Knowing how powerful APOE4 is,” he said, “I can imagine a patient with mild cognitive impairment, who has one or two copies of APOE4, whom I would encourage to get into an antisense oligonucleotide trial now.”
A Resilient Needle in a Neurodegenerative Haystack
Given how common the APOE4 variant is and how substantially it raises Alzheimer’s disease risk, it might seem surprising that scientists knew so little about how it works. It’s clear that APOE is in some way involved with cells’ ability to process amyloid beta, a protein that forms sticky plaques in the brains of people with Alzheimer’s, but the specifics are murky.
To gain insight into the functioning of APOE4 and other disease-linked variants, scientists often try to study similar genes in lab animals like mice—but mice, who live for only a couple years, are of limited value in understanding a disease that usually takes decades to emerge. “Our mouse models are pretty poor,” Greicius said.
That left humans. Of course, Greicius couldn’t just block the APOE4 variant in people and watch what happened—if the APOE4 mutation were simply a weakened version of the normal APOE gene, further disabling it could prove even more devastating. Instead, he needed to search for individuals in whom the gene was naturally disabled: people who carried such dramatic mutations that the gene could not possibly produce a protein, even a partially functional one.
Such unambiguous mutations are vanishingly rare. But with the advent of large-scale genetic datasets, finding such sporadic mutations has become increasingly plausible. With support from the Knight Initiative, Greicius and his colleagues combed through 56,864 genetic profiles from the Alzheimer’s Disease Sequencing Project dataset to uncover seven individuals with disabled APOE genes. It’s a modest group, but, as a whole, they appear to be unexpectedly resistant to both the formation of amyloid beta plaque and the symptoms of Alzheimer’s disease. That’s a strong sign that disabling APOE4 could be a promising strategy for combating the condition.
Only two of the seven individuals with disabled APOE genes had disabled copies of APOE4; the others had severe mutations in APOE3, a variant not associated with increased disease risk. But the two individuals with disabled APOE4 showed a striking pattern: they were both cognitively healthy and showed no evidence of amyloid plaques, even at advanced ages.
One of the subjects was still cognitively healthy and had normal amyloid levels in their spinal fluid into their late 70s, at which point more than two-thirds of APOE4 carriers show significant amyloid buildup. For the other individual, who died at age 90, the lack of amyloid was particularly remarkable: At that age, his brain should have been clogged with the protein, but when scientists inspected his brain after autopsy, they could find no plaque at all.
At first, Greicius had been tentative, given his small sample size, but when he examined these two subjects, he started to see a scientific story form. “With the two of those together, I started feeling a little more confident,” he said. “I think the extremeness of the phenotypes is what carries the story.”
The other five subjects, too, support the overall story: inactivating a copy of APOE didn’t appear to increase their Alzheimer’s risk, either. That suggests that antisense oligonucleotide treatments targeting APOE4 are likely safe, and the first two subjects, with the disabled copies of APOE4, indicate that such treatments could protect against disease.
Still, seven people is a small group on which to base treatment decisions. Greicius says he looks forward to finding more examples of APOE-disabling mutations in the genetic databases. For now, these seven individuals provide a promising picture of the potential benefits of disabling APOE4.