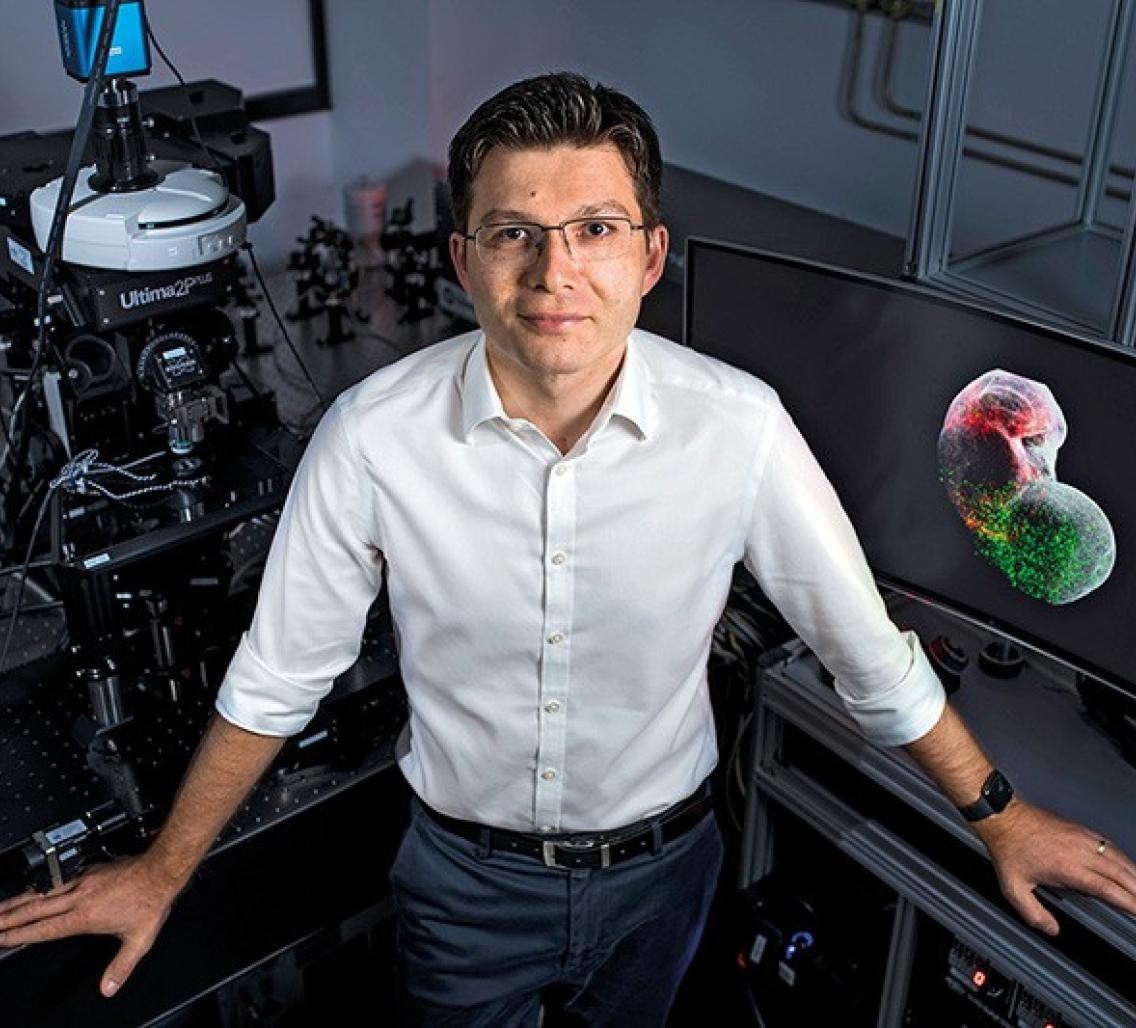
By Charlie Schmidt
3D models of biological tissue that incorporate multiple cell types are the latest tools for understanding human development and disease.
Ever since he was a medical student in Romania, Sergiu Pașca has wanted to understand how connections between cells go awry in the brains of people with psychiatric disorders. Because the living human brain is inaccessible, these conditions could be diagnosed and classified only according to their behavioural symptoms, rather than their underlying biological causes.
In 2017, Pașca took a major step towards his goal. By this time, he was a physician-scientist at Stanford University in California, and using induced pluripotent stem cells (iPS cells) to model various structures in the brain. Cultured from adult skin cells, iPS cells could be prodded in Pașca’s laboratory to grow into 3D spheroids that mimic neuronal tissues such as the frontal cortex. Spheroids are useful for studying the emergence and properties of individual neurons, “but also limited in that we couldn’t use them to study complex interactions involving multiple cell types”, Pașca says.

Sergiu Pașca is a physician-scientist at Stanford University in California.Credit: S. Pașca Lab, Stanford Univ.
 Part of Nature Outlook: Stem cells
Part of Nature Outlook: Stem cells
These interactions are crucial to how the brain gets wired up during development, and Pașca wanted to model them using iPS-cell-derived tissues in a dish. So, he and his team performed an experiment: they combined spheroids from two distinct brain regions involved in higher-order thought processes. And remarkably, the two spheroids fused together, just as they would in the brain of a growing baby. No one had ever witnessed this early developmental process before, and Pașca marvelled at the sight of it. “The cells within the spheroids knew just where to go,” he says. “They started changing their morphology to form synapses and become electrically integrated.”
Pașca coined the term “assembloid” to describe this construct of neural circuits1. As he defines them, assembloids are 3D structures formed from the fusion and functional integration of multiple cell types. And, most important, they mimic the complex cellular interactions from which organs arise in the body.
Assembloids are now at the leading edge of stem-cell research. Scientists are using them to investigate early events in organ development, and as tools for studying not only psychiatric disorders, but other types of disease as well. According to Pașca, assembloids have the advantage of revealing how interactions between different tissues give rise to new cellular properties. For instance, some neurons activate secondary developmental programs required for integration into circuits only after meeting up with the other brain cells they connect to. And by creating assembloids using cells derived from people with particular diseases, researchers should be able to reproduce the inherited pathology of their diseases in a dish. It is hoped that assembloids will lessen the need for laboratory animals, and open doors for high-throughput screening of drugs and chemicals.
The cultures do have limitations: they have no blood supply; they do not contain all the cell types that govern the functioning of real organs in the body; and nor are they exposed to the multiple stressors that organs experience in real life. Still, the ability to model tissue-to-tissue interactions in the lab marks a significant advance, says Avindra Nath, an immunologist and clinical director of the National Institute of Neurological Disorders and Stroke, in Bethesda, Maryland. “This is a big step in the right direction,” he says.
Assembly strategy
During their pivotal 2017 experiment, Pașca and his team coaxed one set of iPS cells to grow into spheroids from the cerebral cortex, and another to form spheroids from a deeper part of the forebrain called the subpallium. Because they did not know the rules that govern self-assembly of circuits in the brain, the researchers could not simply program the different cells to connect. Instead, their aim was to provide an environment in which the cells might perform the necessary processes on their own.
Placing them together at the bottom of a centrifuge tube full of nutrient-rich media did the trick: the tissues combined overnight. The team is now systematically trying to work out how the right cells find each other during the assembly process. They have also moved on to model other connections, including the integration of cells from the cortex and the striatum. Faulty cortical–striatal circuits are implicated in autism spectrum disorder, schizophrenia and other neurodevelopmental conditions.
Takanori Takebe, a research physician at Cincinnati Children’s Hospital Medical Center in Ohio and the Tokyo Medical and Dental University in Japan, uses a similar method to model how complex tissues form in the gut. His research shows that spheroids based on the front and back of the gastrointestinal tract, respectively, will fuse into an assembloid that soon undergoes a remarkable transformation. Precursor cells start to appear at the boundaries where the spheroids connect, and then the growing assembloid sprouts branches containing new cells for adjacent organs2. Takebe says the model system allows him to see, for instance, “how primitive gut tissues give rise to the liver, bile duct, duodenum and pancreas”.
Meanwhile, Kapil Bharti, a cell biologist at the US National Eye Institute in Bethesda, is experimenting with building assembloids by printing cells onto a biodegradable scaffold in 3D. He prints using three different cell types — endothelial cells, pericytes and fibroblasts — that are components of a structure called the choroid, which controls blood flow to the eye3. This bioprinting approach can be tightly controlled, Bharti says, and helps to ensure that the cells stay together during assembly.
Modelling disease
The use of assembloids for modelling diseases is now top priority. In Pașca’s 2017 study, some of the iPS cells came from people with Timothy syndrome, a rare illness resulting from inherited mutations that affect calcium signalling. People with Timothy syndrome have severe cardiac abnormalities that can be fatal if untreated, and survivors often develop autism spectrum disorder and epilepsy. Pașca’s findings suggested that brain pathology in these cases might result from defects in how nerve cells called interneurons travel from the subpallium to the cortex during development.

A cortical–striatal assembloid generated from human pluripotent stem cells.Credit: S. Pașca Lab, Stanford Univ.
Migrating interneurons ordinarily move by inching forwards rather like a caterpillar, covering a distance of about 40 micrometres every few hours. Assembloids generated using iPS cells from people with Timothy syndrome, however, showed a different behaviour: the cells ‘jumped’ more often, but for shorter distances1. “They’re eager to jump, but can only jump a little bit so they get left behind,” Pașca says. Because these cells inhibit the activity of other neurons in the cortex, Pașca says that their faulty migration might cause imbalances in electrical activity that predispose people with Timothy syndrome to neurological disorders. But it was only by studying how cells interact in the assembloid that these peculiar movements were detected.
Bharti is using assembloids to study the underlying causes of and potential therapies for macular degeneration in the eye, which is a leading cause of age-related blindness. By growing assembloids from iPS cells derived from people with the condition, Bharti can pinpoint which eye tissues are most affected by genetic risk factors for the disease. His team recently used assembloids as tools for drug discovery, identifying an antibody against vascular endothelial growth factor as a potential therapy.
Scientists are also starting to rely on assembloids for infectious-disease research. In July, Joseph Gleeson, a neurologist at the University of California, San Diego, used them to explore how SARS-CoV-2 might affect the central nervous system (CNS).
Gleeson cultured iPS-cell-derived cortical brain spheroids, and found they were impervious to SARS-CoV-2 infection. But he also knew that a different cell type in the brain, the pericyte, expresses a surface receptor called angiotensin-converting enzyme 2, to which the virus readily binds. Pericytes have important roles in regulating blood-vessel diameter, and also help to maintain the blood–brain barrier. Mostly originating as neural crest cells during development, pericytes migrate into the cortex as the brain becomes vascularized. When Gleeson and his team combined cortical spheroids with pericytes in the lab, they found that the different cells generated an assembloid that was susceptible to SARS-COV-2 infection4. The virus infected pericytes, as well as another cell type growing in the assembloid, called an astrocyte.
Gleeson speculates that pericytes might serve as viral replication hubs that explain some of COVID-19’s CNS symptoms. His colleague Aaron Carlin, an infectious-disease physician, concurs, adding that infection of pericytes might induce inflammatory cascades “leading to the strokes we so often see in COVID patients in the intensive-care unit”. The assembloid offers benefits over simpler spheroid systems, Gleeson says, because it mimics “the unique aspects of biology that can only be understood and modelled by reconstituting key cellular interactions”.
Increasing complexity
Researchers are developing ever-more complex assembloids. In a study that aims to accelerate progress in understanding the neurological condition motor neuron disease, or amyotrophic lateral sclerosis (ALS), for instance, Pașca’s team last year published an iPS-cell-derived model of the corticospinal tract and its target muscles. The muscles contract in response to stimulation of the nervous tissue, mimicking how motor information travels from brain to muscle5. In addition, Pașca’s team can use a patient’s own cells to generate these assembloids, generating personalized working models to study how circuit defects lead to neuromuscular impairments such as those seen in ALS.
Scientists have a way to go when it comes to creating assembloids that more perfectly mimic real tissues, however. “We’re still missing essential components, especially immune cells that can move freely among the cells used to establish assembloids,” says Jan Brosens, an obstetrics and gynaecology researcher at the University of Warwick in Coventry, UK. He uses assembloids to study endometrial diseases and pregnancy loss6. In the case of neural tissue, Pașca adds, a developing human brain is shaped by sensory experiences that lab-grown tissues do not have access to. Furthermore, scientists can be limited by the number of spheroids that they can combine into a single structure. Beyond a certain size — Pașca suggests four to five components — culture medium cannot penetrate into the assembloids. Lacking a vasculature, the cells could potentially be starved of nutrients.
Still, Pașca stands by the aphorism that all models are wrong, and some are useful. “There’s been important progress in the field in a short period of time,” he says.
Nature 597, S22-S23 (2021)
doi: https://doi.org/10.1038/d41586-021-02628-x
This article is part of Nature Outlook: Stem cells, an editorially independent supplement produced with the financial support of third parties. About this content.
References
- Birey, F. et al. Nature 545, 54–59 (2017).
- Koike, M. et al. Nature 574, 112–116 (2019).
- Bharti, K. et al. Preprint at https://www.researchsquare.com/article/rs-135775/v1(2021).
- Wang, L. et al. Nature Med. https://doi.org/10.1038/s41591-021-01443-1 (2021).
- Andersen, J. et al. Cell 83, 1913–1929 (2020).
- Rawlings, T. M. et al. eLife 10, e69603 (2021).