Toolmakers aim to untangle fundamental challenges in neuroscience

In an ancient Indian fable, a group of blind men sets out to learn about elephants. But each examines a different part of the animal and comes away with a different conclusion: an elephant is like a snake, like a spear, like a wall, like a tree trunk, like a rope.
Neuroscience has long faced a similar challenge, with even fundamental questions such as the number of cell types in the brain producing different answers when viewed through the lenses of genetics, anatomy, circuit connectivity or behavioral function. And unlike the blind men and the elephant, neuroscientists are limited in their ability to directly study the object of their desire: the human brain itself.
But two groups of Stanford researchers are tackling these long-standing problems head on. As part of the Wu Tsai Neurosciences Institute’s Big Ideas in Neuroscience program, they are forging new technologies and connections between disciplines that have the potential to transform the field:
The Neuro-Omics Initiative is bringing to bear new technologies to bridge long-standing gaps in understanding between molecular and systems-level descriptions of the brain, while the Stanford Brain Organogenesis Program is developing new laboratory models of human brain circuits to allow the field to study the human brain’s unique development — and how its complex circuits go awry in neurological and psychiatric disease.
Following successful pilot phases since their launch in 2018, the Wu Tsai Neurosciences Institute is elevating these collaborative initiatives to flagship "Phase 2" Big Idea projects with funding to continue their transformative work over the next three years. Together with research accelerator grants to two other Big Idea pilot projects, the Institute is awarding a total of more than $5.5 million to these ambitious, multi-disciplinary research efforts from funds that include philanthropic support from a small group of forward-looking donors known as the "brain trust."
As part of the Big Ideas program, the initiatives are all committed to sharing their new technologies and data broadly with the scientific community, training others in these new techniques, and advancing the Wu Tsai Neurosciences Institute’s commitment to a diverse and inclusive community.
“These projects are creating tools with the promise to dramatically amplify and accelerate our understanding of the brain and brain diseases and applying them to answer fundamental questions in neuroscience,” said William Newsome, PhD, Harman Family Provostial Professor of Neurobiology and Vincent V.C. Woo Director of the Wu Tsai Neurosciences Institute. “To have this innovation taking place here where Stanford researchers will be the first to benefit is a positive result for our entire neuroscience community. I only wish we could fund even more work like this.”
Neuro-Omics Initiative aims to bridge gaps between molecular and systems neuroscience
A decade-long revolution in gene sequencing technology has recently made it possible to unveil the inner workings of cells in unprecedented detail by comprehensively measuring the patterns of gene activity (transcriptomics) and complement of protein machinery (proteomics) produced by individual cells — and to do so at a scale of whole organs and organisms.
The Neuro-Omics Initiative was launched by Alice Ting, PhD, Liqun Luo, PhD, and Stephen Quake, DPhil, who realized that these big data technologies — paired with modern machine learning — could allow scientists to comprehensively and reliably classify the cells of the brain based on their genetic programming. With these unique molecular signatures, neuroscientists could for the first time confidently examine the same brain cells from many different perspectives: from their local anatomy and physiology to their participation in the large-scale circuit activity that is responsible for the ability to perceive, think, remember, and act.
“Explaining circuit-level behavior at the level of cells and molecules is a fundamental goal of neuroscience,” said Ting, a professor of genetics and of biology and Chan Zuckerberg Biohub Investigator. “If you think about it, this is a necessary step if you want to come up with new therapeutics, which in most cases act on molecules and cells but ultimately must affect circuits and behavior to treat brain disorders.”
“Our goal has been to develop state-of-the-art techniques to answer questions about complex neural tissues that had never before been possible to ask, and to share these tools with the neuroscience community,” added Luo, who is the Ann and Bill Swindells Professor in the Stanford School of Humanities and Sciences, a professor of biology and a Howard Hughes Medical Institute investigator.

The Neuro-Omics team from left to right: Liqun Luo, PhD, (Biology); Alice Ting, PhD, (Biology, Genetics); Lauren O'Connell, PhD, (Biology); Stephen Quake, DPhil, (Bioengineering, Applied Physics); Jure Leskovec, PhD, (Computer Science); and Bo Wang, PhD, (Bioengineering). (Image credit: Wu Tsai Neurosciences Institute)
Joining the effort are a multi-disciplinary team of collaborators, including machine learning expert Jure Leskovec, PhD, evolutionary and organismal biologist Lauren O'Connell, PhD, and bioengineer and developmental biologist Bo Wang, PhD. “We’ve all admired one another’s work for a long time, but the Big Idea program made us think concretely about how to bring our different techniques and perspectives together and spurred us to launch ambitious projects that we wouldn't have done without being asked to think seriously about how we would transform the field,” Luo said.
Demonstrating the power of their approach, the team recently used neuro-omics techniques to crack long-standing questions about the circuitry, function and evolution of a little-studied part of the cerebellum called the deep cerebellar nuclei. Transcriptomic analysis of mouse, chicken, and human brain tissue revealed repeated circuit “motifs” — patterns of brain cells that have been copied and pasted to create additional cerebellar nuclei during the evolution of mammals, and particularly humans. Unexpectedly, given the cerebellum’s traditional role in motor planning, the researchers found that the more recently evolved nuclei projected to brain regions involved in higher cognitive functions, suggesting that the replication of cerebellar circuits could have played a role in the evolution of advanced cognition.
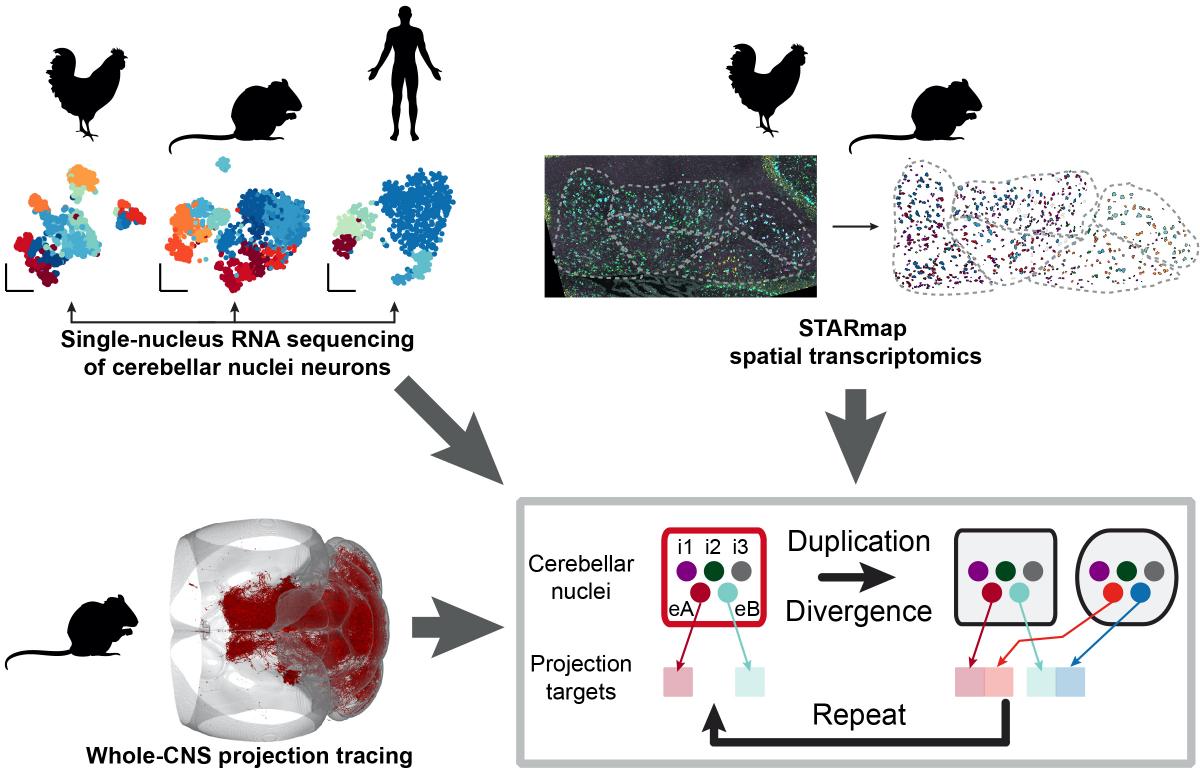
Illustration of neuro-omics techniques showing identification of cell types of the cerebellum, their anatomical positions and brain-wide projections, which together revealed new insights into the evolution of the cerebellar nuclei in chickens, mice, and humans. (Image credit: Kebschull et al, Science, 2020)
“Using transcriptomics, we can compare the roles of different cell types within a given species, but we can also compare similar cell types across different species to gain a new understanding of how the brain evolved," said Quake, who is Lee Otterson Professor in the Stanford School of Engineering, a professor of bioengineering and of applied physics, and co-President of the Chan Zuckerberg Biohub. "This is an area where I’m super excited that these techniques have the potential to profoundly impact the field.”
The team has developed other new technologies to study brain circuits and function across even broader scales, from comprehensive mapping of all synaptic proteins in the intact fly olfactory system to analysis of the specific cells and circuits engaged during fear learning in mice.
With their Phase 2 Big Ideas funding, the Neuro-Omics team plans to go big, expanding beyond classic model organisms like flies and mice into creatures such as flatworms, butterflyfish, poison frogs, sleep lizards, and king quail, with the goal of understanding common factors in the evolution of brain cells and circuits across the tree of life. They also plan to develop and share new tools for sequencing-based circuit analysis to understand the relationship between cell types and the elaborate brain-wide circuits that make possible our ability to think, feel, and dream.
Stanford Brain Organogenesis Program to Study Complex Human Brain Circuits in the Lab
The Stanford Brain Organogenesis Program, led by Sergiu Pasca, MD, and Karl Deisseroth, MD, PhD, aims to create and share a new technological platform to enable scientists to directly study the development of the human brain, and how the brain’s self-wiring goes awry in neurological and psychiatric disease.
“Animal models have been very helpful, but ultimately most would agree that we need better models of the human brain to translate fundamental discoveries to our patients in the clinic,” said Pasca, the Bonnie Uytengsu and Family Director of the Stanford Brain Organogenesis Program and an associate professor of psychiatry and behavioral sciences. “Fortunately, the call for Big Ideas came just as a set of powerful technologies were being developed across campus that could make this possible for the first time.”
Pasca’s group had developed an approach to transform skin cells of patients and healthy volunteers into cellular aggregates that resemble specific brain regions. In 2017, his lab showed that these 3D cultures, also known as organoids, could be grown together to form miniature, functional human brain circuits — which Pasca dubbed assembloids. “We realized that this series of technological advances would allow us to access previously inaccessible aspects of human brain development for the first time — and ultimately to create a new field of human neuroscience at the molecular, cellular and circuit level,” Pasca said.
For example, Pasca’s team recently used this assembloid platform to create working models of the human brain circuits involved in motor disorders like ALS — combining spheroids of human cortical, spinal, and muscle tissue in lab dishes and allowing them to naturally form functional connections. The group now has the ability to model a variety of neural circuits involved in schizophrenia, addiction, and other disorders by combining spheroids mimicking over a dozen different brain regions.
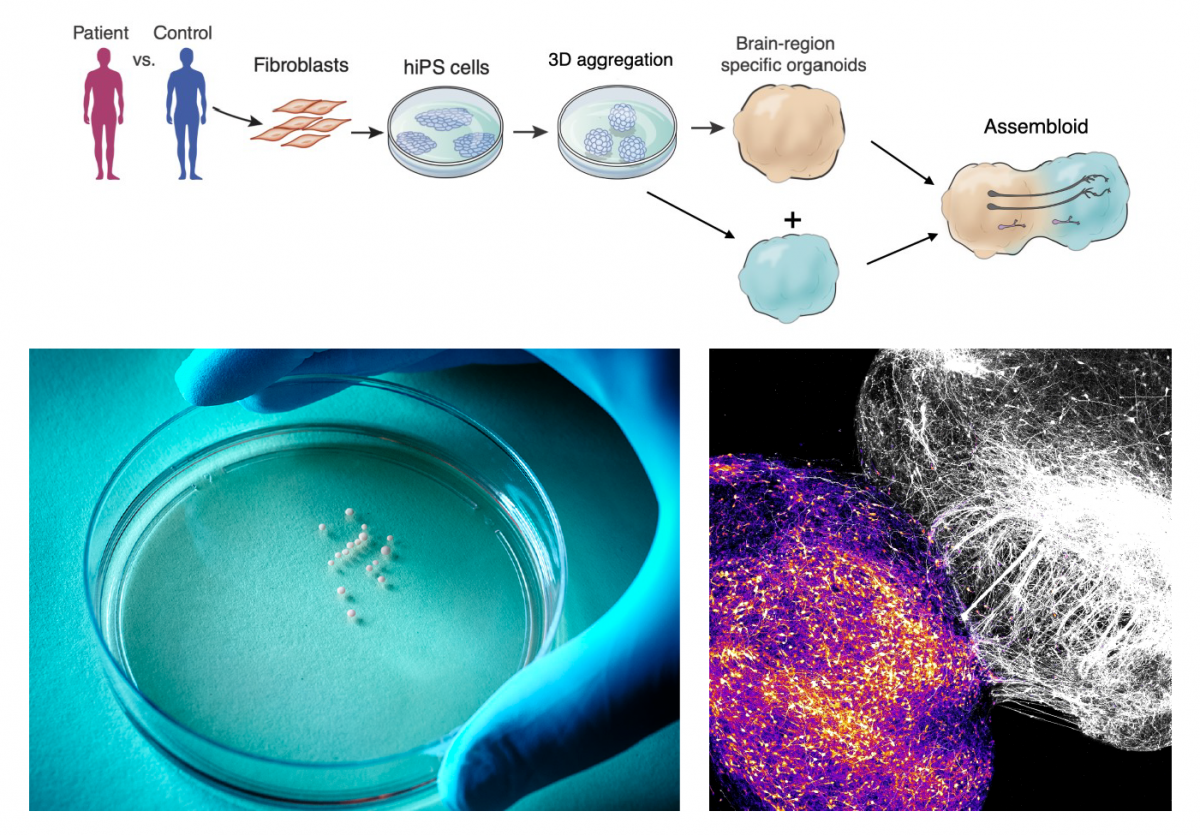
Spheroids of human brain cells can be derived from patient skin cells (left) then combined into “assembloids” that recreate the development of brain circuits involved in neuropsychiatric disorders, such as the cortico-striatal circuits involved in disorders of motivation (right). (Image credit: Pasca lab, Wu Tsai Neurosciences Institute)
Pasca realized early on that these ever-more complex models of human brain tissue would require a suite of new tools to monitor and interact with the assembloids as their circuits develop and integrate with one another, which can take many months.
He found an eager partner in Deisseroth, who is renowned for developing lab-based technologies to enable precise control and observation of brain circuits, but as a practicing psychiatrist, longs for better approaches to studying human brain circuits. “Psychiatric diseases pose an incalculable burden on society, but because of the ethical and technical challenges of studying the developing human brain, we lag behind other fields of medicine in our understanding of the origins of these essentially human conditions,” said Deisseroth, who is D.H. Chen Professor of Bioengineering and of Psychiatry and Behavioral Sciences at Stanford and a Howard Hughes Medical Institute investigator.
The duo recruited collaborators from across Stanford schools and departments, each of whom contributed their own powerful technologies for interacting with and studying lab-grown brain tissue, including flexible electronics developed by chemical engineer Zhenan Bao, PhD; nanoscale sensors developed by chemist Bianxiao Cui, PhD; biocompatible compounds developed by materials scientist Sarah Heilshorn, PhD; and genetically encoded voltage sensors developed by bioengineer Michael Lin, PhD.
“As an engineer, I make things and I build things, but I want to have a purpose for the materials we make and the devices we build,” said Bao, whose group has created skinlike, stretchable electronic meshes that can cradle growing assembloids like shrink-wrap while also recording the electrical and neurochemical communications between cells and stimulating them to promote their development. “What's better than understanding how the brain works and helping to solve neuropsychiatric diseases that have puzzled people for so many years without having good treatment methods?"
The team also recruited bioethicist Hank Greely, JD, Deane F. and Kate Edelman Johnson Professor of Law, to advise the group on potential ethical issues surrounding creating even very simple human brain circuits in the lab — the topic of a recent National Academy of Sciences report to which Greely and Newsome contributed — and to develop a set of guidelines for the growing field.

The Stanford Brain Organogenesis Program team from left to right: Karl Deisseroth, MD, PhD, (Bioengineering, Psychiatry and Behavioral Sciences); Michael Lin, PhD, (Bioengineering, Neurobiology); Zhenan Bao, PhD, (Chemical Engineering); Sarah Heilshorn, PhD, (Materials Science and Engineering); Bianxiao Cui, PhD, (Chemistry); Hank Greely, JD, (Law); and Sergiu Pasca, MD, (Psychiatry and Behavioral Sciences). (Image credit: Avery Krieger for the Wu Tsai Neurosciences Institute)
The new Phase 2 Big Ideas funding will be the time to put all the technology they’ve developed so far to the test, the team says — creating a platform to study the development of a wide array of human neural circuits, testing their discoveries against ex vivo human tissue samples, and exploring their ability to integrate into the brains of living mice.
“We’ve been able to do this because all these world leaders in bioengineering, chemistry, and neurobiology all work within a couple minutes’ walk of one another,” Deisseroth said. “It's hard to imagine this happening anywhere other than at Stanford, or without the impetus of the Wu Tsai Neuroscience Institute’s push to develop big ideas with the potential to fundamentally change the field.”
Research Accelerators to focus on Early Brain Development, Mechanisms of Plant-based Medicine
The Wu Tsai Neurosciences Institute is also announcing two-year Research Accelerator grants to two other 2018 Big Idea pilot projects:
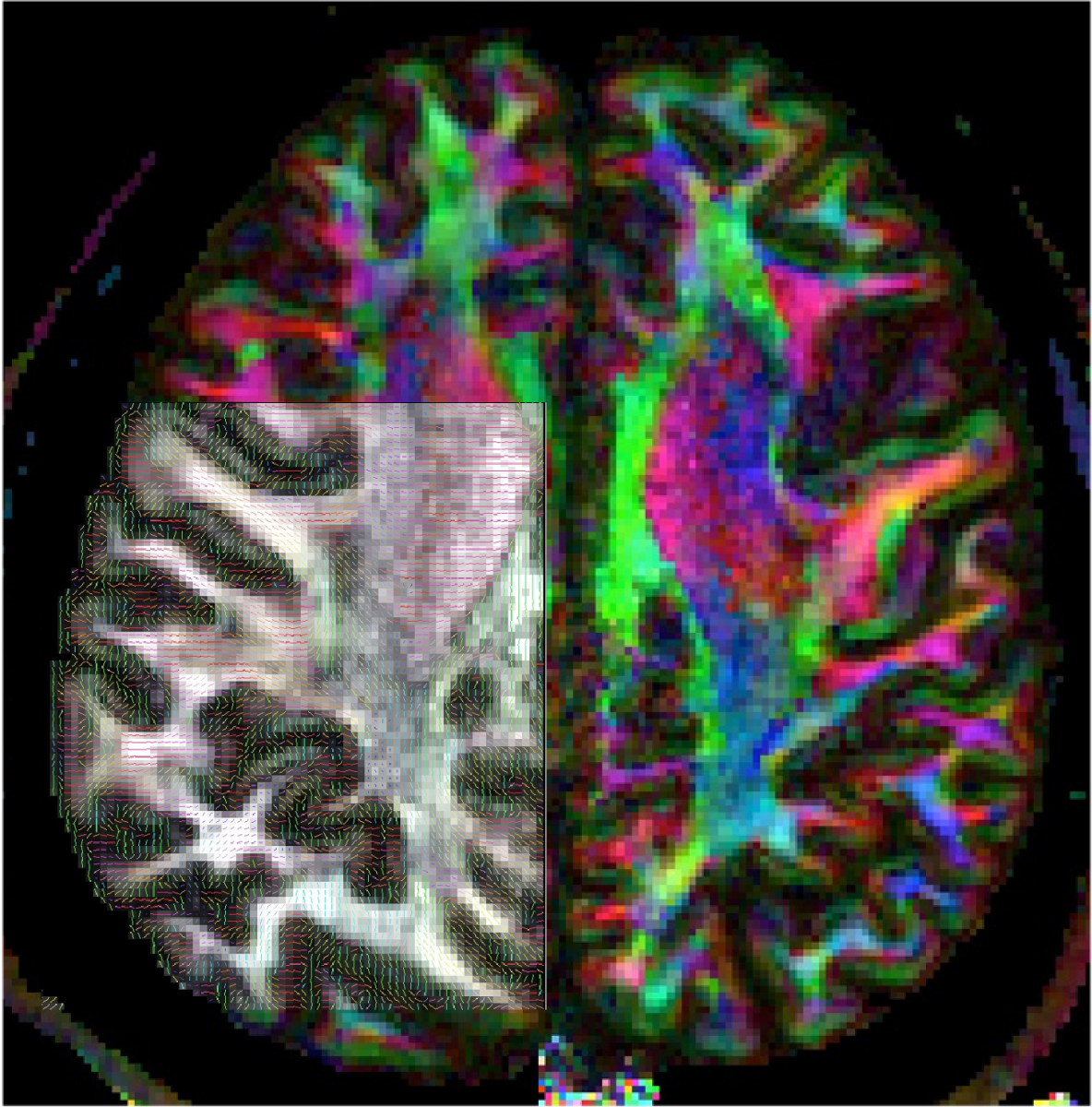
Improved MRI image quality enables mapping of nerve fiber patterns not only in the white matter but also in the grey matter of the cerebral cortex. (Image credit: Neurodevelopment Initiative, Wu Tsai Neurosciences Institute)
The Neurodevelopment Initiative, led by psychologist Kalanit Grill-Spector, PhD, and MRI physicist Jennifer McNab, PhD, is developing new tools and techniques for newborn and infant magnetic resonance imaging (MRI), with the goal of better understanding early human brain development and improving screening for neurodevelopmental abnormalities in the first months of life.
In particular, the group aims to directly compare the unique microscopic anatomy of the infant brain with how these features are reflected in MR images, to gain a better understanding of the unique biology of the developing brain. In service of this goal, the team has developed a new library of custom protocols and code designed for imaging and analyzing the infant brain, which they are making freely available to the scientific and medical community. The group will also use the accelerator funds to develop and test a new adjustable MRI coil for researchers at the Stanford Center for Neurobiological Imaging that is designed like a car seat to make infant brain imaging more comfortable and reliable and can be adapted to infants’ changing head size.
“I have been scanning children and comparing them to adults for 15 years, but the baby brain is just very different. It’s not just a smaller version of the adult brain,” said Grill-Spector, a professor of psychology. “For example, the conventional contrast between gray matter and white matter appears inverted in the infant MRI because the myelin that produces this contrast is still developing. This means we’ve needed to create whole new protocols and data analysis software for infant MRI, which we hope will be valuable for the field.”
“To help interpret the neurodevelopmental changes we observe, we have also developed new approaches to directly compare MRI with advanced histology in the same ex vivo tissue samples,” added McNab, an associate professor of radiology. “Gaining a better understanding of what each MRI pixel represents in terms of microstructural features such as the density and organization of myelin, glia and cell bodies will help advance our understanding of this critical stage of development.”

Kalanit Grill-Spector, PhD, (Psychology) and Jennifer McNab, PhD, (Radiology) lead the Neurodevelopment Initiative at the Wu Tsai Neurosciences Institute. (Images courtesy of the researchers.)
The NeuroPlant Initiative aims to identify new neurological drugs by understanding natural compounds evolved by plants to influence animal nervous systems. (Image credit: NeuroPlant Initiative, Wu Tsai Neurosciences Institute)
The NeuroPlant Initiative, led by Thomas Clandinin, PhD, Miriam Goodman, PhD, and Seung Yon (Sue) Rhee, PhD, has created a platform to study the vast number of chemicals plants use to influence animal behavior, with the goal of understanding the biological mechanisms underlying the natural and plant-derived medical products used by 80 percent of humanity and enabling the discovery of new pharmaceutical drugs to treat brain disorders.
“Many established pharmaceuticals are derived from plant compounds — aspirin from willow bark, colchicine from the autumn crocus, and so on,” said Clandinin, the Shooter Family Professor in the Wu Tsai Neurosciences Institute and a professor of neurobiology. “But there is a huge space of medicinal plants where there's real evidence that they work but we don’t yet understand the specific compounds or biological mechanisms.”
“We’re excited to match the first set of bioactive plant compounds we’ve identified to the animal receptors they interact with, and to disseminate the high-throughput platform we’ve established broadly so others can join us in this effort,” said Goodman, the Mrs. George A. Winzer Professor in Cell BIology and chair of the Department of Molecular and Cellular Physiology.
“This will set the stage to address some really cool biological questions about what's going on here in this evolutionary chemical warfare between plants and animals and how it can be used to advance both scientific discovery and human medicine,” added Rhee, a senior staff member in the Plant Biology Department at the Carnegie Institution for Science.

Seung Yon (Sue) Rhee, PhD, (Carnegie Institution for Science); Miriam Goodman, PhD, (Molecular and Cellular Physiology); and Thomas Clandinin, PhD, (Neurobiology), lead the NeuroPlant Initiative at the Wu Tsai Neurosciences Institute. (Images courtesy of the researchers.)
Big Ideas in Neuroscience Initiatives aim to advance field through collaboration, diversity and education
Newsome and the Wu Tsai Neurosciences Institute Executive Committee founded the Big Ideas in Neurosciences program as the Institute’s flagship grant program following a famous series of dinners in which researchers across campus who shared an interest in the brain came together to discuss how the Institute could best leverage its cross-disciplinary scope to move the field of neuroscience forward. The institute awarded its first round of seven Big Ideas grants in 2014, and later advanced three of these transformative projects to flagship "phase 2" collaborations focused on brain rejuvenation, neurotechnology and decision making.
The Institute awards its Big Ideas in Neuroscience grants not only based on their potential to unravel knotty problems in neuroscience, but for their ability to build bridges between disciplines, strengthen the Stanford neuroscience community and contribute to the training of the next generation of multi-disciplinary scientists — including a focus on inclusion of underrepresented and historically marginalized groups.
“We’ve always said that we want these Big Idea teams to bring together ideas from a wide range of fields and disciplines,” Newsome said. “They become more than the sum of their parts, which is what’s needed to give them a shot to accomplish together things that they'd have no hope of accomplishing apart.”
Full affiliations of Big Idea Initiative lead investigators:
Karl Deisseroth, MD, PhD, is D.H. Chen Professor in the departments of Bioengineering and of Psychiatry and Behavioral Sciences in the Stanford schools of Engineering and of Medicine, and a Howard Hughes Medical Institute investigator. He is a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute.
Sergiu Pasça, MD, is the Bonnie Uytengsu and Family Director of the Stanford Brain Organogenesis Program at the Wu Tsai Neurosciences Institute and associate professor in the Department of Psychiatry and Behavioral Sciences in the Stanford School of Medicine. He is a member of Stanford Bio-X, the Stanford Center for Sleep Sciences and Medicine, the Stanford Maternal & Child Health Research Institute (MCHRI) and the Wu Tsai Neurosciences Institute and a Stanford ChEM-H faculty fellow.
Liqun Luo, PhD, is Ann and Bill Swindells Professor in the Department of Biology in the Stanford School of Humanities and Sciences and professor by courtesy in the Department of Neurobiology in the Stanford School of Medicine. He is a member of Stanford Bio-X, the Stanford Cancer Institute and the Wu Tsai Neurosciences Institute and a Stanford ChEM-H faculty fellow.
Stephen Quake, DPhil, is the Lee Otterson Professor in the Department of Bioengineering in the Stanford Schools of Engineering and of Medicine and professor in the departments of Applied Physics and (by courtesy) of Physics in the Stanford School of Humanities and Sciences, and is co-President of the Chan Zuckerberg Biohub. He is a member of Stanford Bio-X, the Stanford Cardiovascular Institute, the Stanford Cancer Institute, the Stanford Diabetes Research Center and the Wu Tsai Neurosciences Institute.
Alice Ting, PhD, is professor in the departments of Biology and Genetics and (by courtesy) of Chemistry, in the Stanford School of Humanities and Sciences. She is a member of Stanford Bio-X, the Stanford Maternal & Child Health Research Institute (MCHRI), the Stanford Cancer Institute and the Wu Tsai Neurosciences Institute, and a Stanford ChEM-H faculty fellow.
Kalanit Grill-Spector, PhD, is a professor in the Department of Psychology in the Stanford School of Humanities and Sciences and a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute.
Jennifer McNab, PhD, is an associate professor in the Department of Radiology in the Stanford School of Medicine. She is a member of Stanford Bio-X, the Stanford Maternal & Child Health Research Institute (MCHRI) and the Wu Tsai Neurosciences Institute. She is also director of industry collaborations in the Department of Radiology and co-director of the Neurosciences Visualization Community Laboratory at the Wu Tsai Neurosciences Institute.
Thomas Clandinin, PhD, is Shooter Family Professor in the Wu Tsai Neurosciences Institute, and professor in the Department of Neurobiology in the Stanford School of Medicine. He is a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute.
Miriam Goodman, PhD, is the Mrs. George A. Winzer Professor in Cell Biology and chair of the Department of Molecular and Cellular Physiology in the Stanford School of Medicine. She is a member of Stanford Bio-X and chair of the Wu Tsai Neurosciences Institute Interdisciplinary Scholars Program.
Seung Yon (Sue) Rhee, PhD, is a Senior Staff Member in the Plant Biology Department at the Carnegie Institution for Science and associate professor (by courtesy) in the Department of Biology in the Stanford School of Humanities and Sciences, and a member of the Wu Tsai Neurosciences Institute.


